Reactive metabolites can cause drug-induced toxicity and attrition. They are short-lived, electrophilic species formed during Phase I drug metabolism that can covalently bind to macromolecules. While defensive mechanisms usually inactivate them, high doses or individual vulnerabilities can overwhelm these defenses and cause organ or immune toxicity. Common precursors are functional groups like alkyl halides and sulfonates that undergo oxidation to form reactive species like epoxides, imines, and quinones. Understanding reactivity and how to avoid or minimize reactive metabolite formation could improve drug safety.







![S
O
O O
Alkyl
Alkyl halides and sulfonates
[Br,I,Cl]
Electrophilic esters
SNAr electrophiles
N
A
[F,Cl,Br] N
A
OSO2R
Wide variety of structures! (EWG= electronwithdrawing group)
[F,Cl]
EWG
O
O
Ar
O
O
O N
H
Oxiranes and aziridines
O
SO2
Michael acceptors
Awareness/avoidance of intrinsic reactivity
[F ,C l]
E W G
Very useful presentation at SCI
Conference, March 25, 2011, on
”Designing Safer Medicines in
Discovery”
Title: ChEMBL & Structural Alerts
By Francis Atkinson
Chemogenomics Group
EMBL – EBI, Hinxton
http://www.soci.org/News/Fine-Safer-Medicines-2011-Papers.aspx
10](https://image.slidesharecdn.com/increaseddrugsafety-avoidingreactivemetabolites-210120161630/75/Increased-drug-safety-avoiding-reactive-metabolites-10-2048.jpg)







![Quinoids comprise a major category of RMs
O
[C,N,O]
R
OH
[C,N,O]
R
OH
[C,N,O]
Nu
R
Nu
P450 or
other
enzyme
In this large group the electrophilic system consists of a quinone, a quinone-
imine, a quinone-diimine, or the corresponding methides, the quinone
methides (quinomethanes) and quinone-imine methides. Only the para isomers
are depicted below.
Testa et al. (Drug Disc Today 2012, 17, 549) have analysed the literature and
conclude: “A markedly greater source of worry and potential toxicity is seen
with redox reactions, most significantly with the formation of quinones,
quinonimines, quinonimides and quinone-diimines, which accounted for 40%
of all toxic and/or reactive metabolites identified in this work.”
In addition, from the abstract of a review (Monks et al. Current Drug Metab
2002): Quinones are ubiquitous in nature and constitute an important class of naturally occurring
compounds found in plants, fungi and bacteria… For example, the quinones of polycyclic aromatic
hydrocarbons are prevalent as environmental contaminants and provide a major source of current
human exposure to quinones. ... . Quinones are oxidants and electrophiles, and the relative
contribution of these properties to quinone toxicity is influenced by chemical structure,
in particular substituent effects.](https://image.slidesharecdn.com/increaseddrugsafety-avoidingreactivemetabolites-210120161630/75/Increased-drug-safety-avoiding-reactive-metabolites-18-2048.jpg)

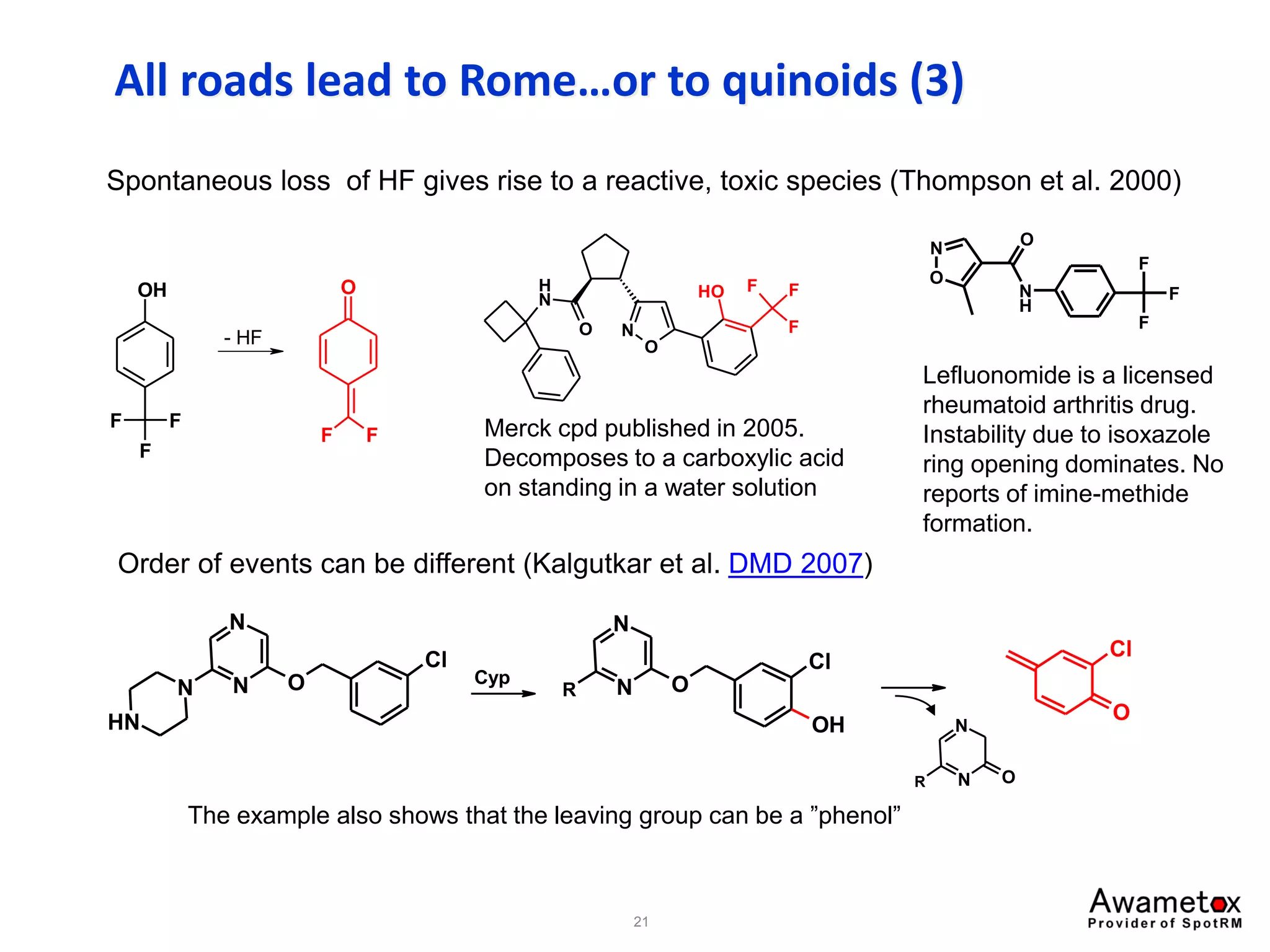
![All roads lead to Rome…or to quinoids (2)
Quinone methides (quinomethanes) and quinone-imine methides.
Thompson et al. studied the phenol below (Toxicology 2001, 160, 197).
OH
O
S
O
O
O
[O,N]
H
H
[N,O]
[O,N]
O
H
H
- H2O
Oxid.
O
H O
O
H
O
H
When the benzylic alcohol forms
a sulfate (via SULT enzymes)
the elimination is even faster
The sulfates of the
corresponding 4-
alkoxy-benzylic
alcohols would also
be quite reactive.
O
O
S
O
O
O
R
20](https://image.slidesharecdn.com/increaseddrugsafety-avoidingreactivemetabolites-210120161630/75/Increased-drug-safety-avoiding-reactive-metabolites-20-2048.jpg)