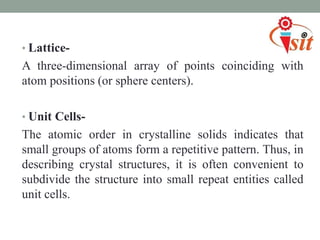
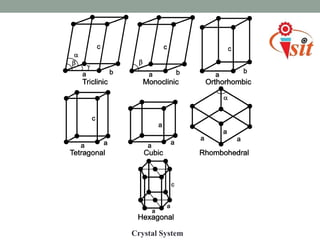
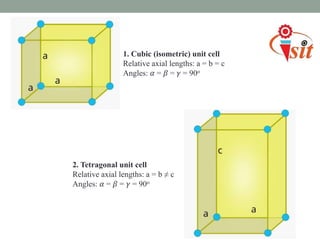
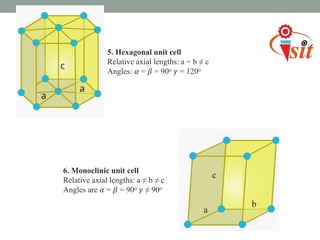
There are 7 crystal systems based on the symmetry and shape of the unit cell:
1. Cubic system has equal lattice parameters and 90 degree angles.
2. Tetragonal system has equal a and b parameters and 90 degree angles.
3. Orthorhombic system has unequal parameters and 90 degree angles.
4. Rhombohedral system has equal parameters and non-90 degree angles.
5. Hexagonal system has equal a and b parameters, non-equal c, and 90 and 120 degree angles.
6. Monoclinic system has unequal parameters and one 90 degree angle.
7. Triclinic system has unequal parameters and no 90 degree angles.