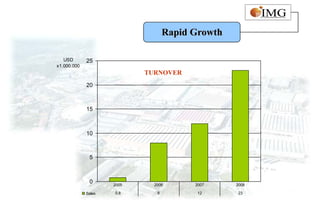
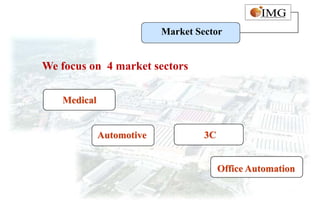
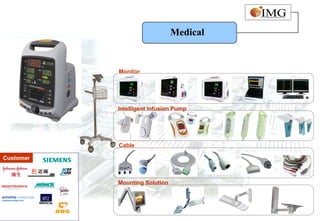
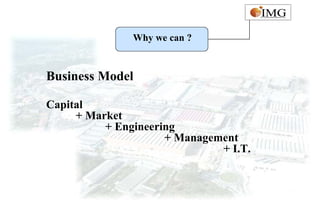
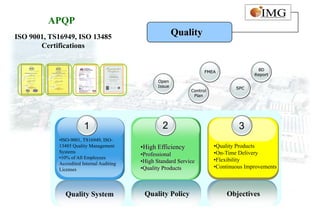
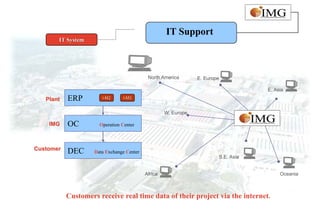
This document provides an overview of IMG Medical Group, a manufacturing group focused on medical device components. It summarizes IMG's mission to deliver high quality medical device manufacturing through engineering expertise and information technology. It outlines IMG's rapid growth, comprehensive manufacturing and R&D capabilities across multiple market sectors including medical, automotive, 3C, and office automation. Key details include IMG's proprietary IT systems, quality management systems, project management approach, and manufacturing resources that enable end-to-end solutions with