Title: An Overview of Contract Research Organizations (CROs)
Description:
In today’s fast-evolving pharmaceutical landscape, **Contract Research Organizations (CROs)** serve as the invisible backbone behind successful drug development and regulatory approval processes. This PowerPoint presentation offers a detailed, well-structured, and student-friendly overview of CROs—from their origin and purpose to their staff roles, key responsibilities, organizational structure, services offered, and real-world challenges.
Perfect for students, researchers, and industry newcomers, this PPT simplifies the complex world of clinical outsourcing into understandable segments.
---
### 📖 What This PPT Covers:
#### 1. **Introduction to CROs**
The presentation begins with a simple but powerful introduction to what a CRO is. Defined as a professional service organization that supports pharmaceutical companies, foundations, or government bodies in clinical research, CROs have become essential in reducing costs, accelerating research timelines, and offering specialized expertise. It also discusses how CROs operate by taking over trial planning, site management, regulatory coordination, and data analysis.
#### 2. **History of CROs**
From their humble beginnings in the 1940s with firms like Charles River Laboratories to their current global presence, CROs have expanded from offering niche services to becoming comprehensive, full-service providers. The presentation traces this journey through key milestones in CRO development—highlighting how they evolved from support labs to strategic partners in clinical trials.
#### 3. **Objectives of CROs**
Here, the core goals of CROs are broken down:
* Efficient outsourcing in clinical trials
* Biostatistics and data integrity
* Supervision in quality control
* Streamlined risk analysis
* Standardization and archiving
These objectives reflect the growing need for speed, accuracy, and reliability in today’s drug development ecosystem.
---
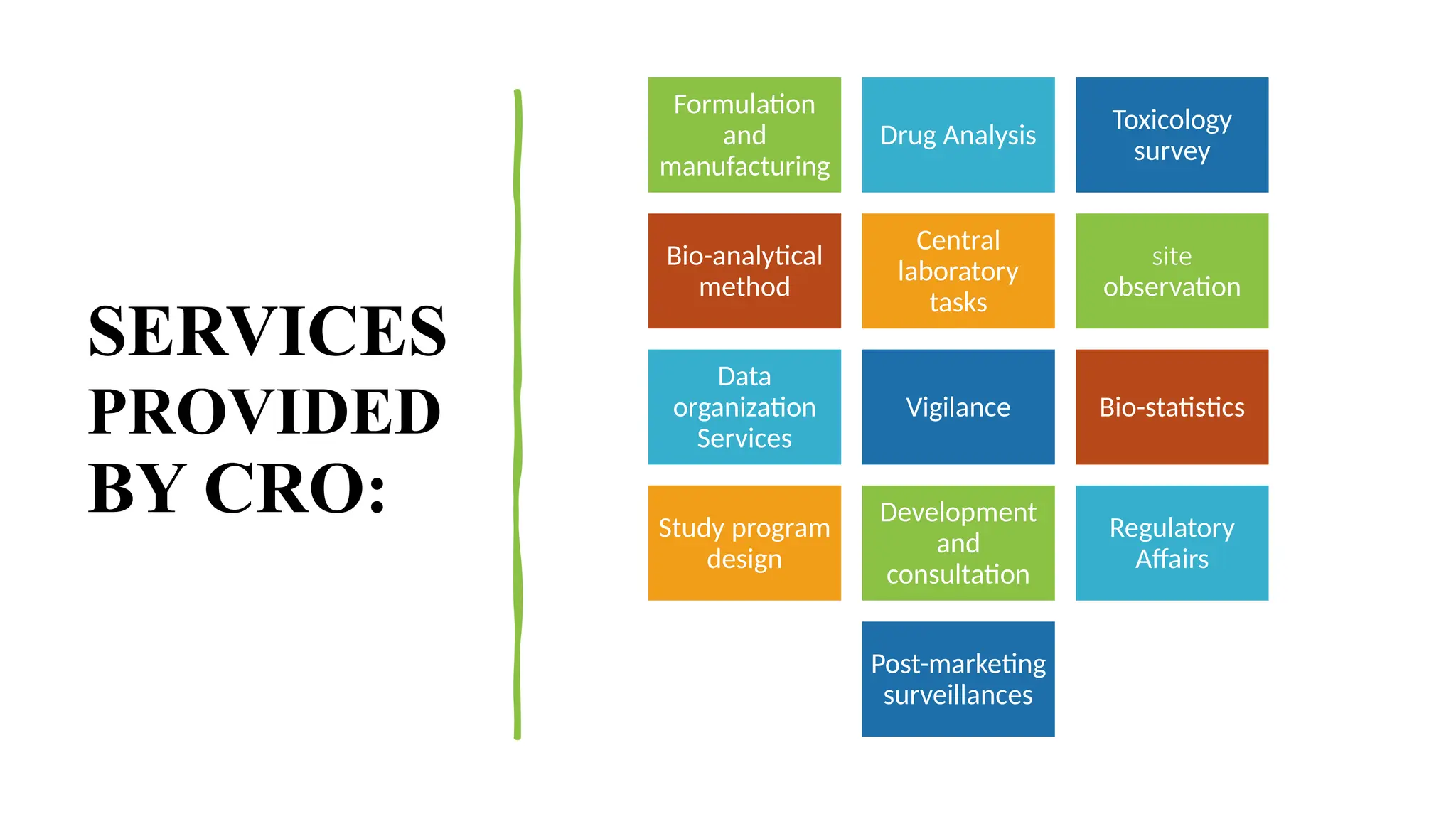
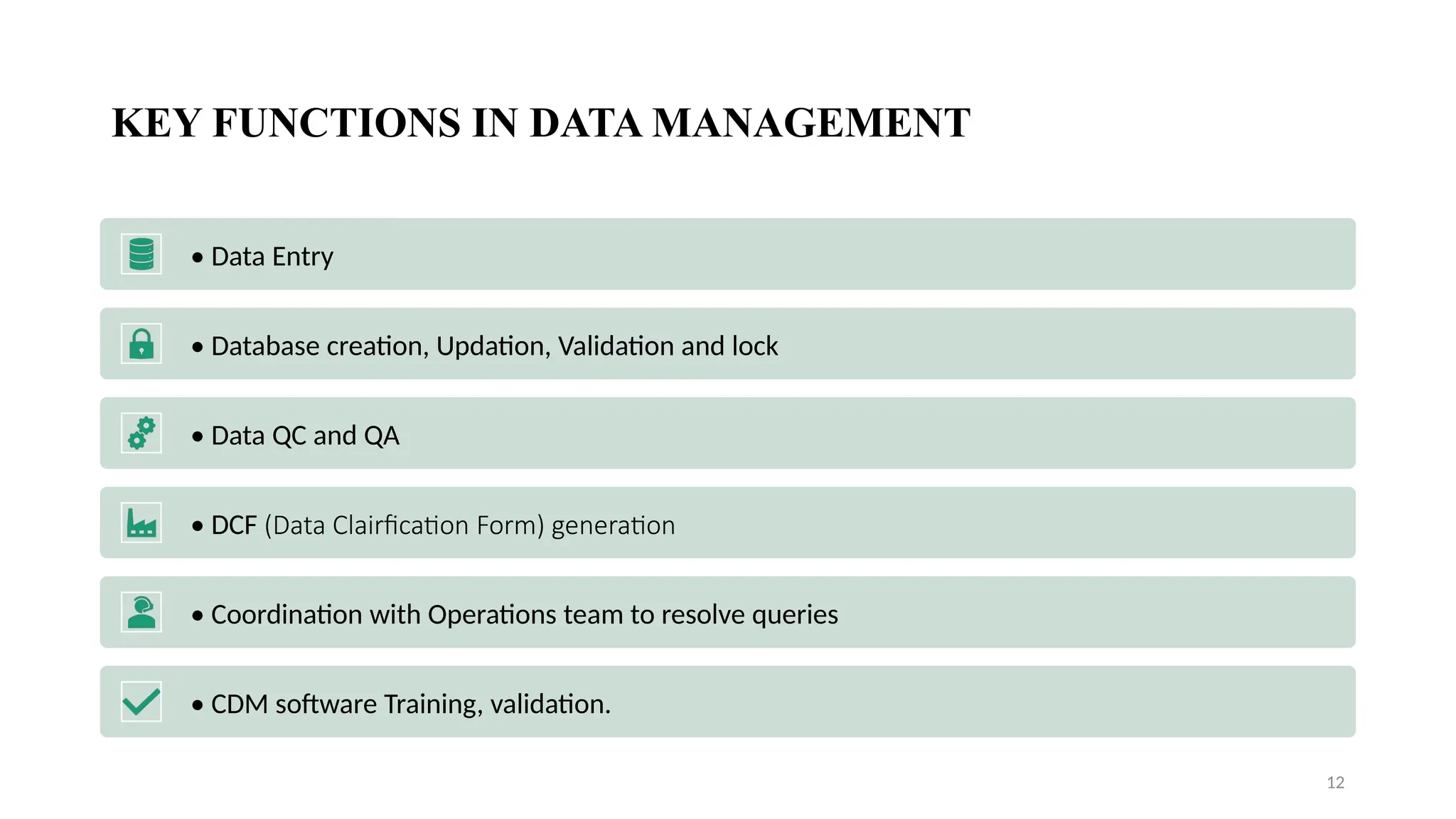
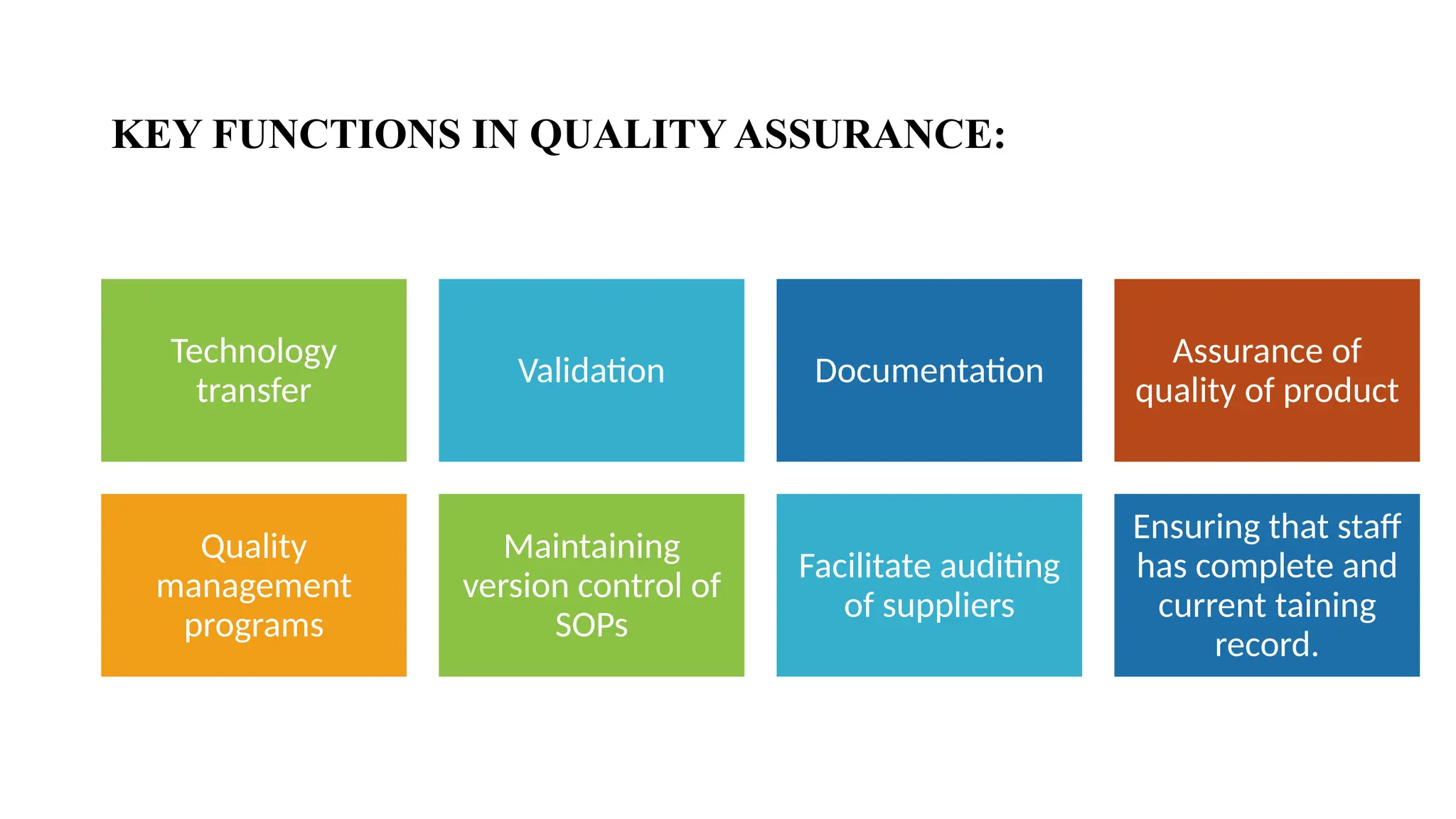
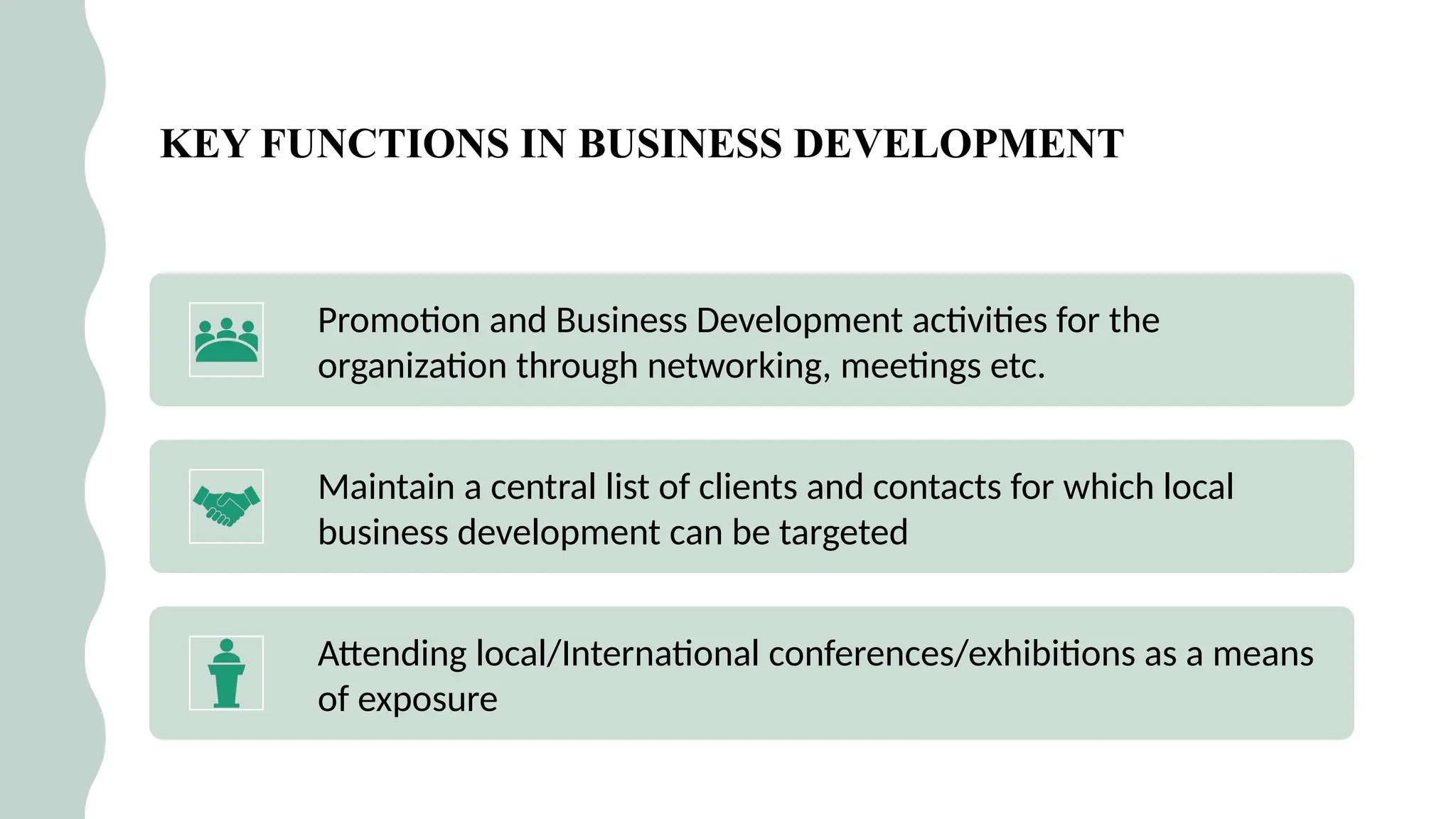
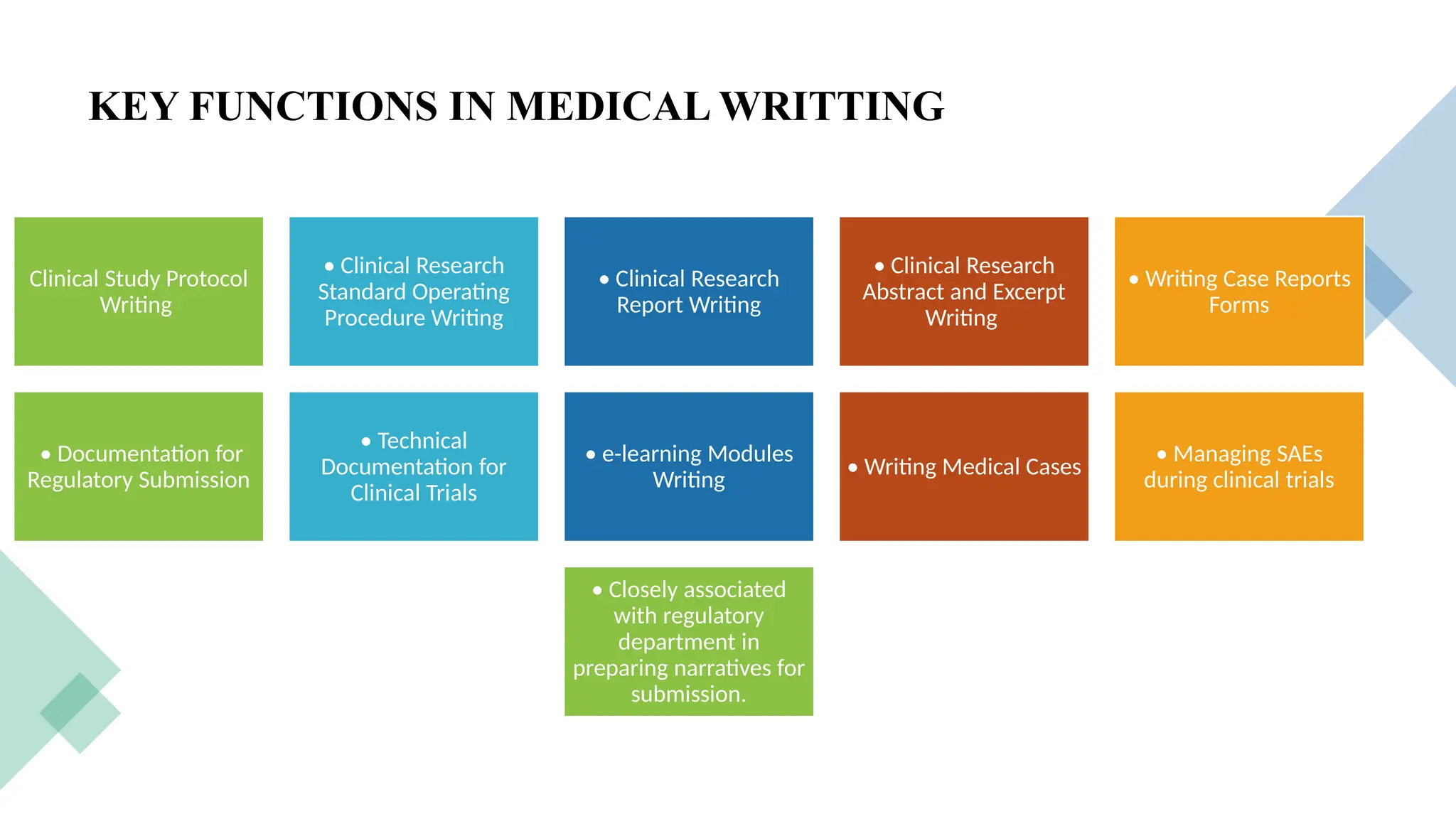
### 🛠️ Services Provided by CROs
This section showcases the wide range of services CROs offer, which go beyond trial execution. They include:
* Preclinical and clinical trial management (Phases I–IV)
* Data collection, entry, and statistical analysis
* Regulatory submissions and correspondence
* Medical writing and documentation
* Pharmacovigilance and post-market surveillance
* Quality control and audits
Each service is introduced in student-friendly language, supported with clear examples.

















![27
[1] Samiran Nundy, M. Chir, Chandra M. Gulhati, A new colonialism conducting clinical trials in
India. The New England Journal of Medicine, 352(16), 2005, 1633- 1636
[2] Biswas P, Biswas AK, Setting standards for proactive pharmacovigilance in India: The way
forward, Indian journal of pharmacology, 39, 2007, 124-128.
[3] Murti Y, Verma P, Pathak D, Awareness of the risk factors in volunteers of clinical trials. The
Pharma Review, 6, 2009. 68-74.
[4] John L. LaMattina, The impact on mergers of pharmaceutical R & D, Nature reviews drug
discovery, 10, 2011, 559-560.](https://image.slidesharecdn.com/contractresarchorganizationppt-250527082618-c7b919fa/75/An-overview-contract-research-organization-27-2048.jpg)
