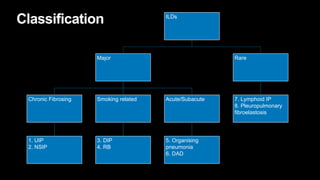
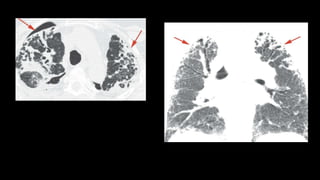
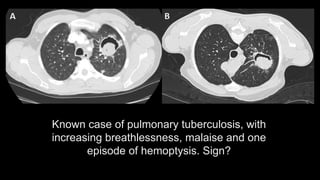
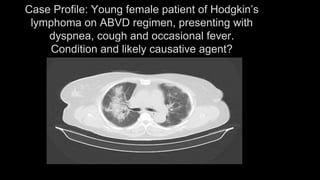
1. The document discusses various interstitial lung diseases (ILDs), their classification, diagnostic criteria and patterns seen on imaging.
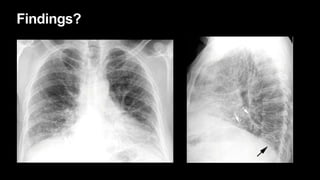
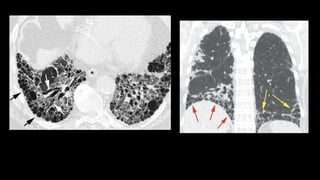
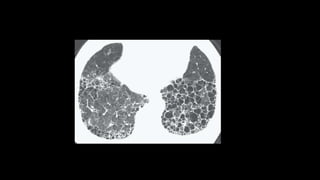
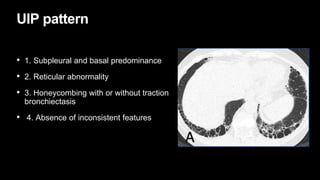
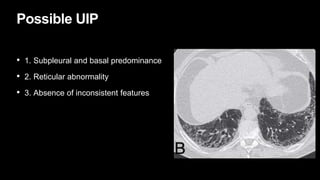
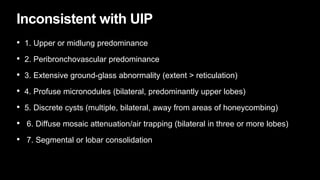
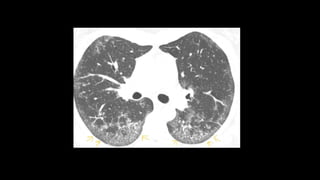
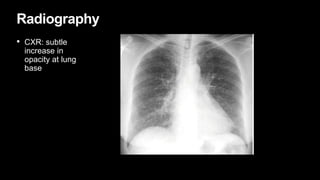
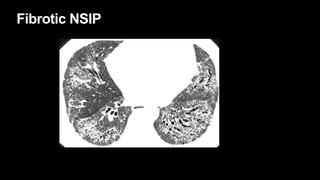
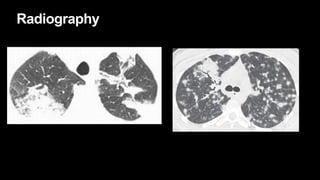
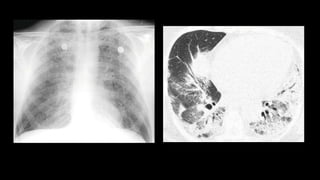
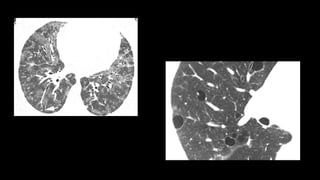
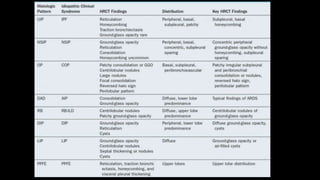
2. It describes usual interstitial pneumonia (UIP) and idiopathic pulmonary fibrosis (IPF) as the most common ILD, seen in older patients with progressive breathing symptoms. Imaging may show honeycombing and reticulation.
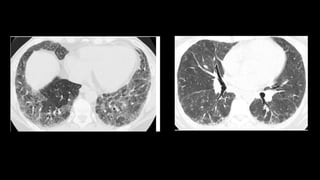
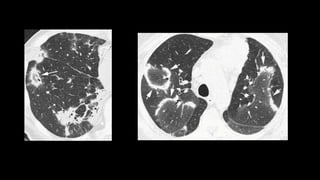
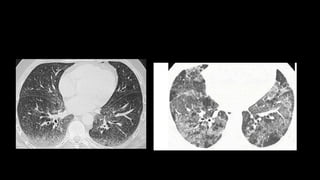
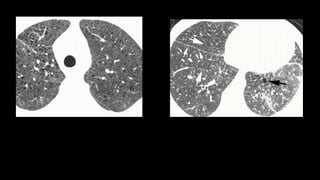
3. Non-specific interstitial pneumonia (NSIP) is less common than UIP and presents at a younger age. It responds well to steroids. Imaging shows ground glass opacity or reticulation without honeycombing.