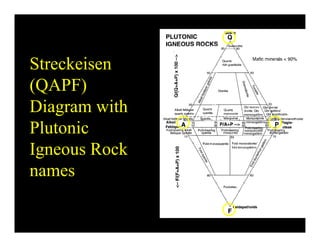
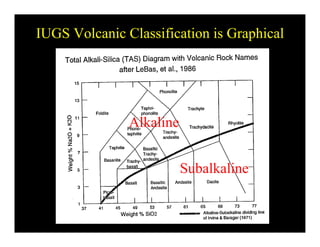
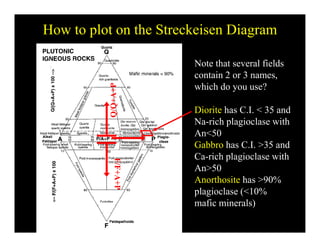
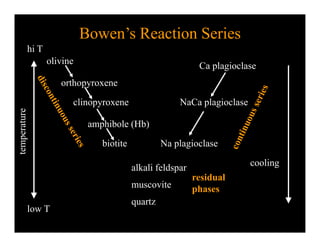
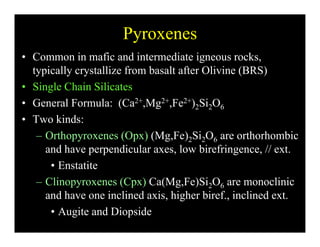
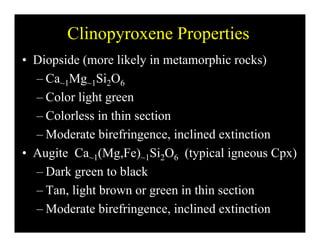
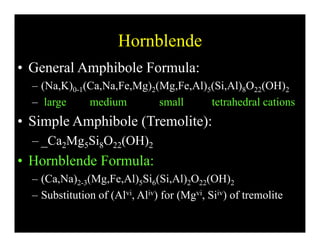
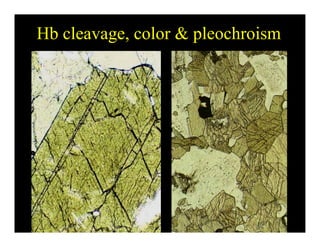
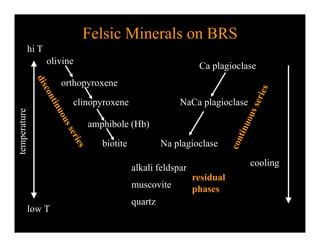
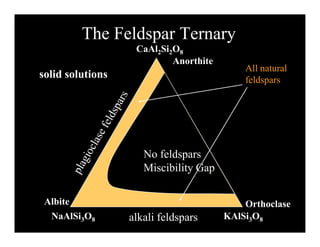
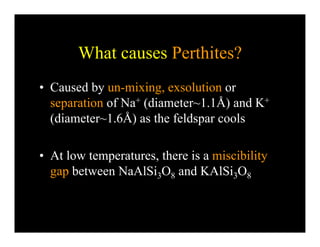
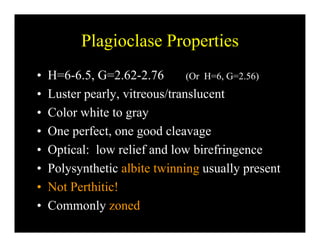
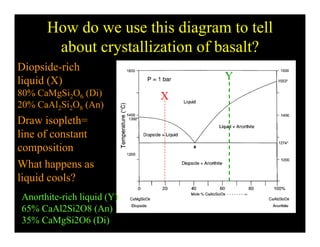
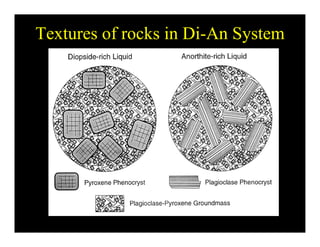
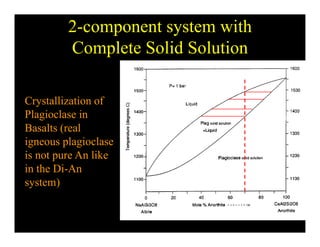
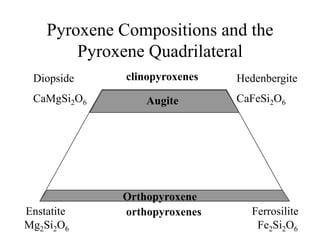
This document provides an overview of igneous rocks and their classification. It discusses that igneous rocks crystallize from magmas, which can be felsic, intermediate, or mafic depending on their silica content. Igneous rocks are classified based on mineral content using diagrams like the QAPF diagram. The ordering of mineral crystallization from cooling magma is explained by Bowen's reaction series. Common igneous minerals like feldspars, pyroxenes, amphiboles, and micas are described in terms of their composition, properties, and where they occur in the crystallization sequence. Phase diagrams provide additional insight into crystallization beyond Bowen's series.