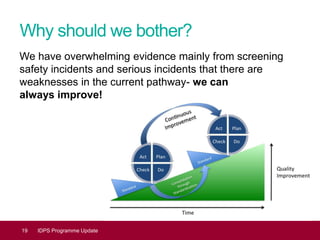
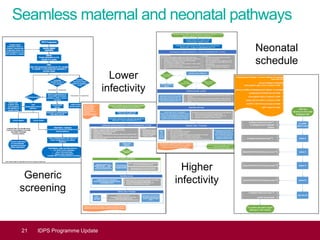
This document provides an update on the Infectious Diseases in Pregnancy Screening (IDPS) Programme in the UK. It discusses the aims of the programme, which include enabling early detection and treatment of infections in pregnancy to reduce mother-to-child transmission. It summarizes screening activity data which shows high uptake rates of over 99% for HIV, hepatitis B, and syphilis screening. It also discusses efforts to improve laboratory quality, establish screening standards and outcomes data, and provide education resources to professionals and the public. Specific updates are provided on actions relating to HIV, syphilis, hepatitis B, and developing seamless maternal and neonatal pathways between screening and immunization programs.