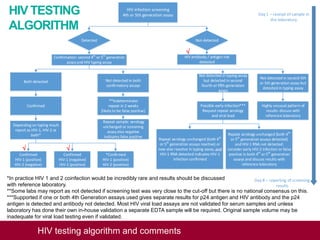
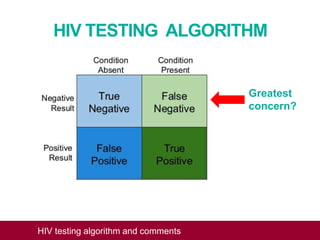
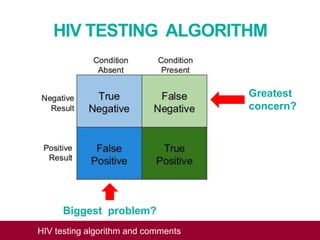
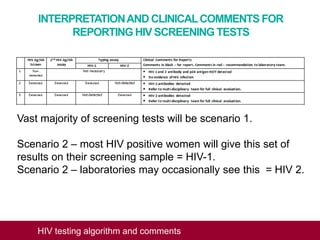
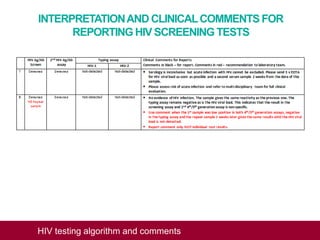
This document outlines an algorithm and provides comments for interpreting and reporting results from HIV screening tests. It describes the typical screening and confirmation testing process and provides guidance on interpreting various result patterns. It also includes questions and discussion points about challenging result scenarios and sample storage requirements. The goal is to help standardize the interpretation and reporting of HIV screening test results.