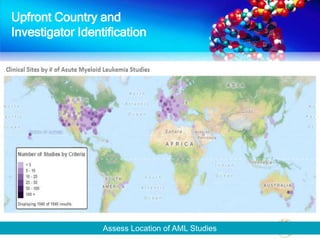
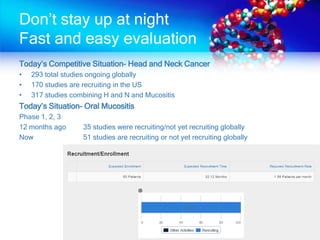
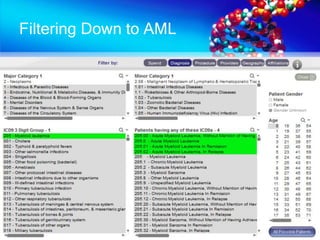
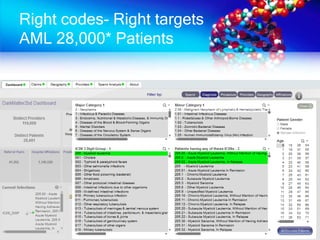
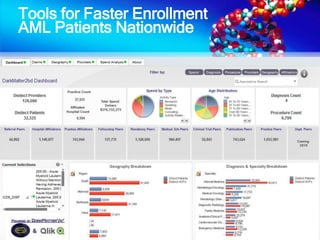
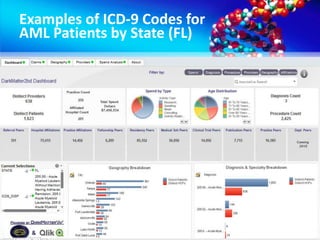
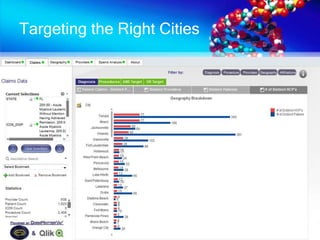
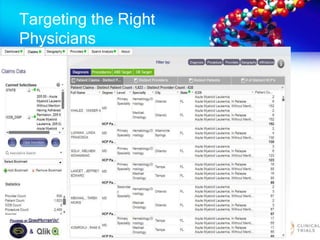
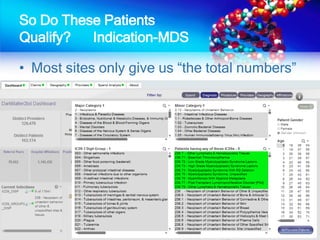
The document discusses patient recruitment strategies in oncology trials, emphasizing the use of technology and data to improve enrollment rates. It highlights the challenges in recruiting patients for clinical trials and the effectiveness of various tools, such as heat mapping and electronic health records, to identify and target suitable candidates. The document advocates for incorporating these tools in the early planning stages of trial execution to enhance enrollment efficiency and overcome industry hesitance to adopt new methods.