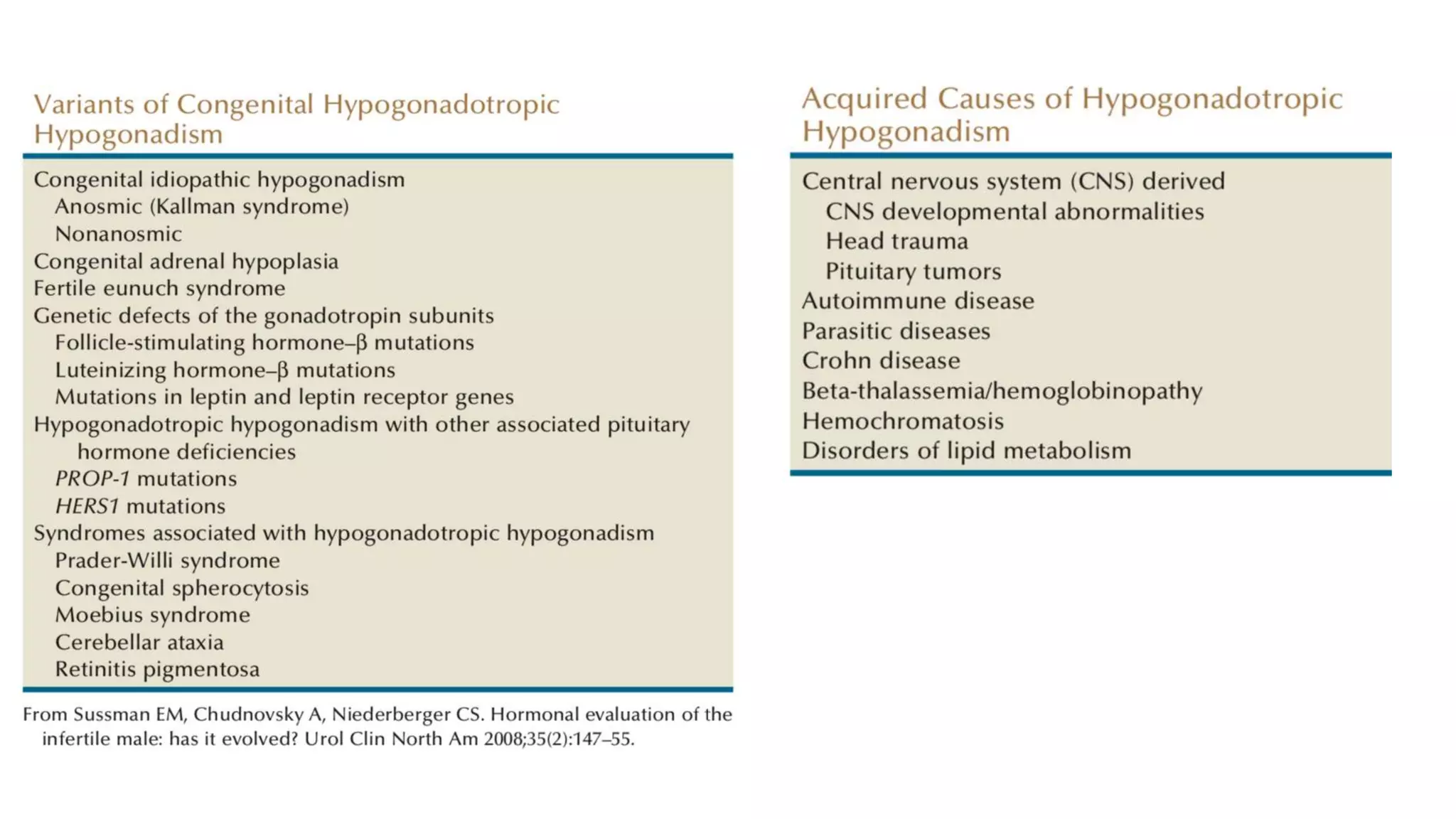
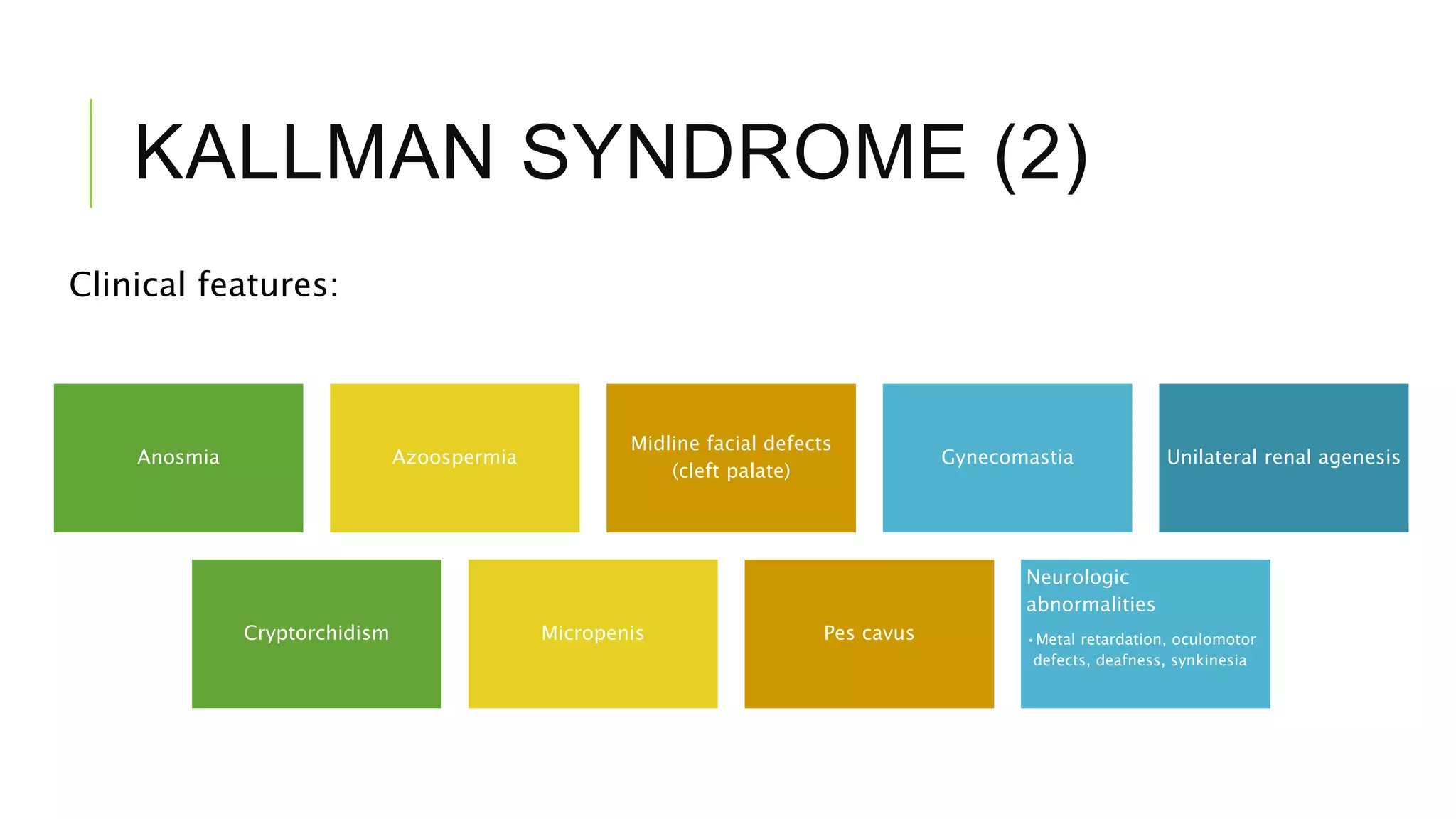
Hypogonadism is characterized by impaired testicular function and can affect sperm production and testosterone synthesis. It has three main categories: primary hypogonadism due to testicular failure, secondary (hypogonadotropic) hypogonadism caused by insufficient gonadotropin secretion, and androgen insensitivity. Hypogonadotropic hypogonadism is rare and can be congenital or acquired. Kallmann syndrome results from GnRH deficiency due to failed embryonic migration and causes anosmia and azoospermia. Prader-Willi syndrome is caused by chromosome abnormalities and results in obesity, short stature, and cryptorchidism. Treatment of hypogonadotropic hyp









![DIAGNOSTIC OF HH (3)
A suspected tumour requires imaging [computed tomography (CT) or
magnetic resonance imaging (MRI)] of the sella region and a complete
endocrine work-up
Normal androgen levels and subsequent development of secondary
sex characteristics (in cases of onset of hypogonadism before
puberty) and a eugonadal state can be achieved by androgen
replacement alone](https://image.slidesharecdn.com/hypogonadotropichypogonadismekoindra-190801041221/75/Hypogonadotropic-Hypogonadism-12-2048.jpg)











