Hukum-hukum gas Pertemuan 1 pendidikan kimia
•Download as PPT, PDF•
0 likes•2 views
Hukum hukum gas komplit
Report
Share
Report
Share
Recommended
More Related Content
Similar to Hukum-hukum gas Pertemuan 1 pendidikan kimia
Similar to Hukum-hukum gas Pertemuan 1 pendidikan kimia (20)
GAS LAWS AND GENERAL GAS EQUATION (CHARLES, BOYLES AND AVOGADRO)

GAS LAWS AND GENERAL GAS EQUATION (CHARLES, BOYLES AND AVOGADRO)
Chemistry - Chp 14 - The Behavior of Gases - PowerPoint

Chemistry - Chp 14 - The Behavior of Gases - PowerPoint
More from heruchristianto
More from heruchristianto (6)
Sosialisasi Visi, Misi, Tujuan Pend. Kimia-2023.pptx

Sosialisasi Visi, Misi, Tujuan Pend. Kimia-2023.pptx
Recently uploaded
God is a creative God Gen 1:1. All that He created was “good”, could also be translated “beautiful”. God created man in His own image Gen 1:27. Maths helps us discover the beauty that God has created in His world and, in turn, create beautiful designs to serve and enrich the lives of others.
Explore beautiful and ugly buildings. Mathematics helps us create beautiful d...

Explore beautiful and ugly buildings. Mathematics helps us create beautiful d...christianmathematics
https://app.box.com/s/7hlvjxjalkrik7fb082xx3jk7xd7liz3TỔNG ÔN TẬP THI VÀO LỚP 10 MÔN TIẾNG ANH NĂM HỌC 2023 - 2024 CÓ ĐÁP ÁN (NGỮ Â...

TỔNG ÔN TẬP THI VÀO LỚP 10 MÔN TIẾNG ANH NĂM HỌC 2023 - 2024 CÓ ĐÁP ÁN (NGỮ Â...Nguyen Thanh Tu Collection
Recently uploaded (20)
Python Notes for mca i year students osmania university.docx

Python Notes for mca i year students osmania university.docx
General Principles of Intellectual Property: Concepts of Intellectual Proper...

General Principles of Intellectual Property: Concepts of Intellectual Proper...
Z Score,T Score, Percential Rank and Box Plot Graph

Z Score,T Score, Percential Rank and Box Plot Graph
Seal of Good Local Governance (SGLG) 2024Final.pptx

Seal of Good Local Governance (SGLG) 2024Final.pptx
Role Of Transgenic Animal In Target Validation-1.pptx

Role Of Transgenic Animal In Target Validation-1.pptx
Measures of Dispersion and Variability: Range, QD, AD and SD

Measures of Dispersion and Variability: Range, QD, AD and SD
Beyond the EU: DORA and NIS 2 Directive's Global Impact

Beyond the EU: DORA and NIS 2 Directive's Global Impact
Explore beautiful and ugly buildings. Mathematics helps us create beautiful d...

Explore beautiful and ugly buildings. Mathematics helps us create beautiful d...
Energy Resources. ( B. Pharmacy, 1st Year, Sem-II) Natural Resources

Energy Resources. ( B. Pharmacy, 1st Year, Sem-II) Natural Resources
Asian American Pacific Islander Month DDSD 2024.pptx

Asian American Pacific Islander Month DDSD 2024.pptx
TỔNG ÔN TẬP THI VÀO LỚP 10 MÔN TIẾNG ANH NĂM HỌC 2023 - 2024 CÓ ĐÁP ÁN (NGỮ Â...

TỔNG ÔN TẬP THI VÀO LỚP 10 MÔN TIẾNG ANH NĂM HỌC 2023 - 2024 CÓ ĐÁP ÁN (NGỮ Â...
Hukum-hukum gas Pertemuan 1 pendidikan kimia
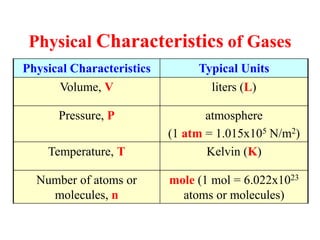
- 1. Physical Characteristics of Gases Physical Characteristics Typical Units Volume, V liters (L) Pressure, P atmosphere (1 atm = 1.015x105 N/m2) Temperature, T Kelvin (K) Number of atoms or molecules, n mole (1 mol = 6.022x1023 atoms or molecules)
- 2. Pressure and volume are inversely related at constant temperature. PV = K As one goes up, the other goes down. P1V1 = P2V2 Boyle’s Law “Father of Modern Chemistry” Robert Boyle Chemist & Natural Philosopher Listmore, Ireland January 25, 1627 – December 30, 1690
- 3. Boyle’s Law: P1V1 = P2V2
- 4. Boyle’s Law: P1V1 = P2V2
- 5. Volume of a gas varies directly with the absolute temperature at constant pressure. V = KT V1 / T1 = V2 / T2 Charles’ Law Jacques-Alexandre Charles Mathematician, Physicist, Inventor Beaugency, France November 12, 1746 – April 7, 1823
- 6. Charles’ Law: V1/T1 = V2/T2
- 7. Charles’ Law: V1/T1 = V2/T2
- 8. At constant temperature and pressure, the volume of a gas is directly related to the number of moles. V = K n V1 / n1 = V2 / n2 Avogadro’s Law Amedeo Avogadro Physicist Turin, Italy August 9, 1776 – July 9, 1856
- 10. At constant volume, pressure and absolute temperature are directly related. P = k T P1 / T1 = P2 / T2 Gay-Lussac Law Joseph-Louis Gay-Lussac Experimentalist Limoges, France December 6, 1778 – May 9, 1850
- 11. The total pressure in a container is the sum of the pressure each gas would exert if it were alone in the container. The total pressure is the sum of the partial pressures. PTotal = P1 + P2 + P3 + P4 + P5 ... (For each gas P = nRT/V) Dalton’s Law John Dalton Chemist & Physicist Eaglesfield, Cumberland, England September 6, 1766 – July 27, 1844
- 12. Dalton’s Law
- 13. Water evaporates! When that water evaporates, the vapor has a pressure. Gases are often collected over water so the vapor pressure of water must be subtracted from the total pressure. Vapor Pressure
- 14. Differences Between Ideal and Real Gases Obey PV=nRT Always Only at very low P and high T Molecular volume Zero Small but nonzero Molecular attractions Zero Small Molecular repulsions Zero Small Ideal Gas Real Gas