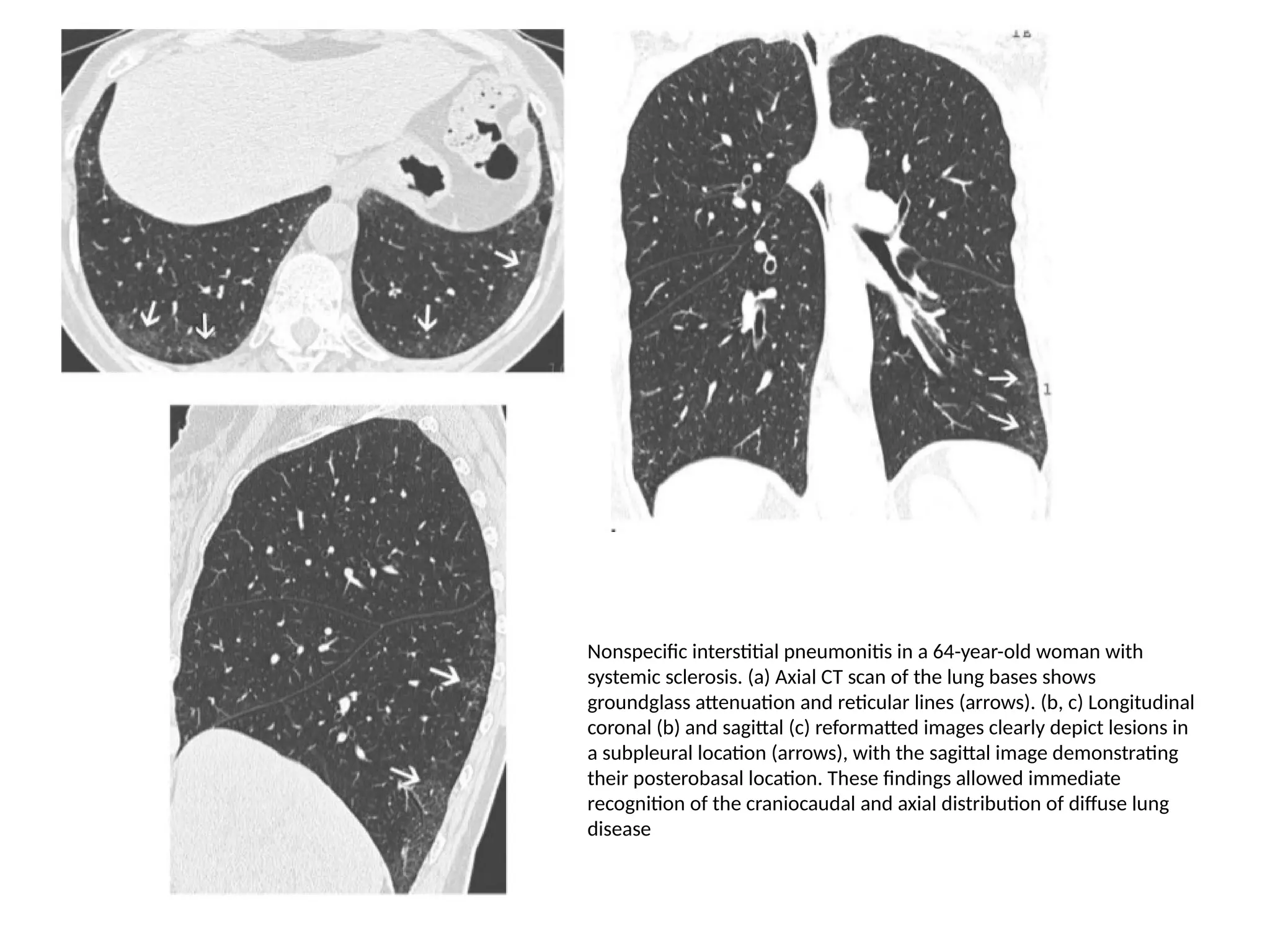
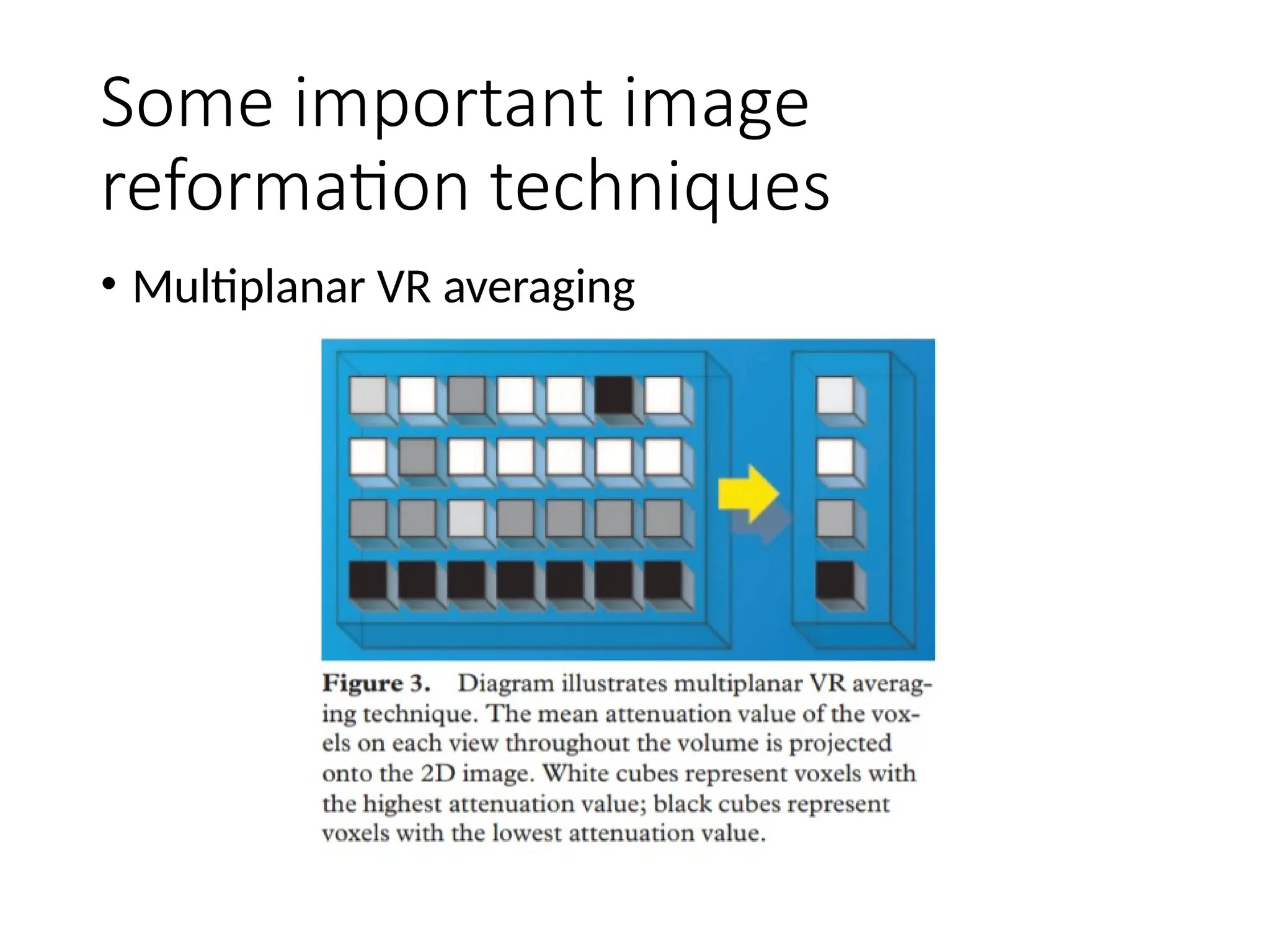
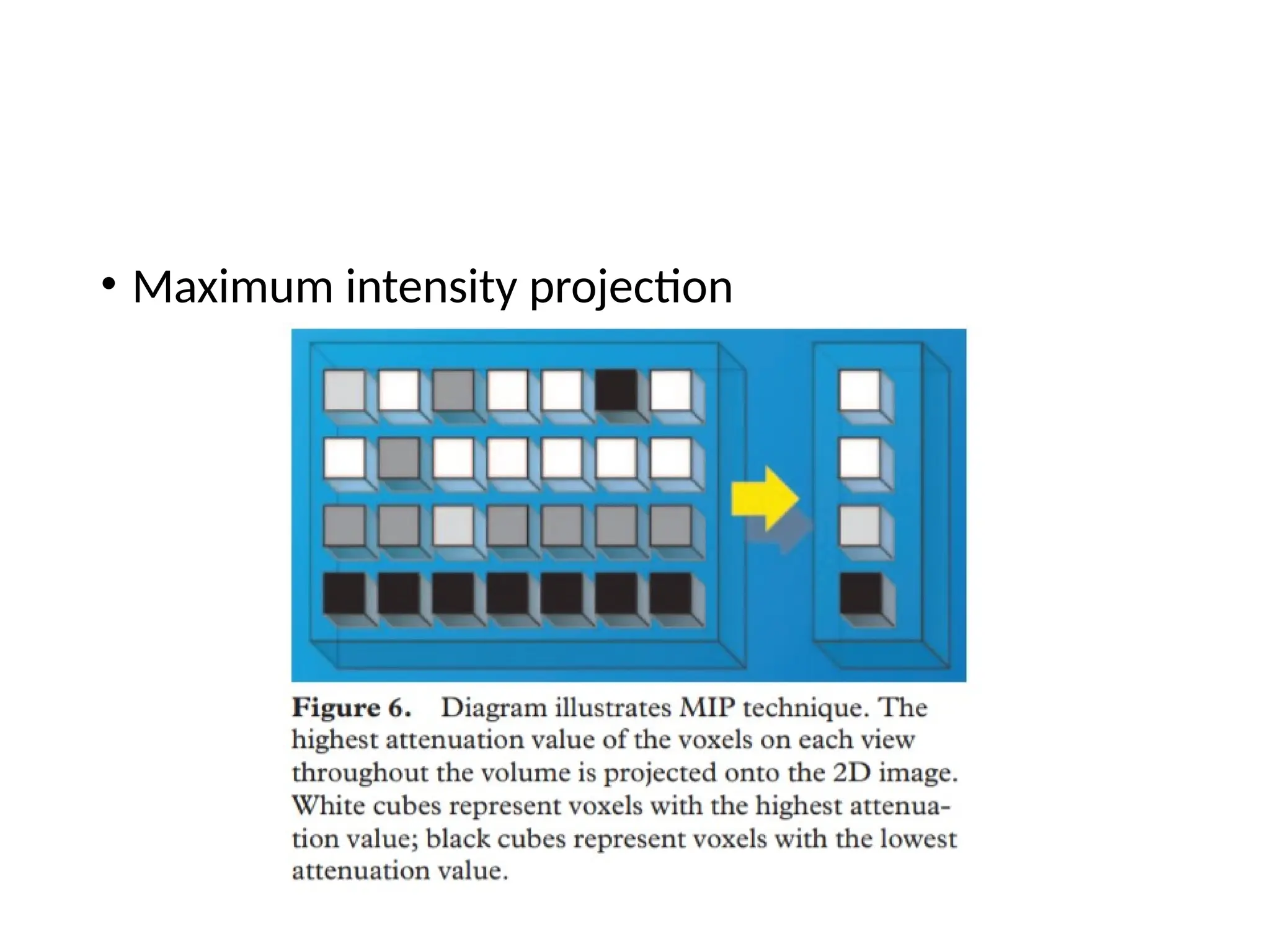
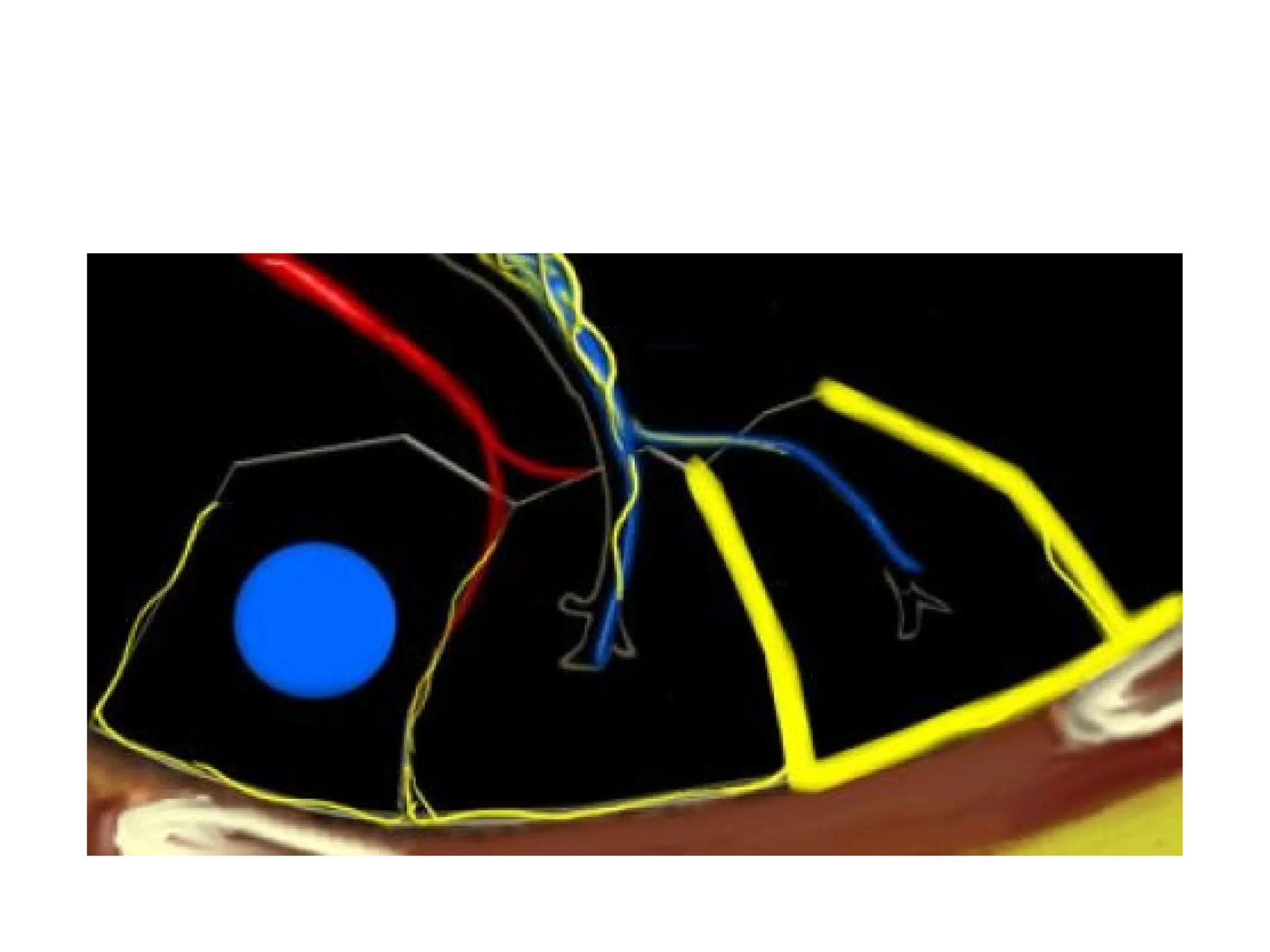
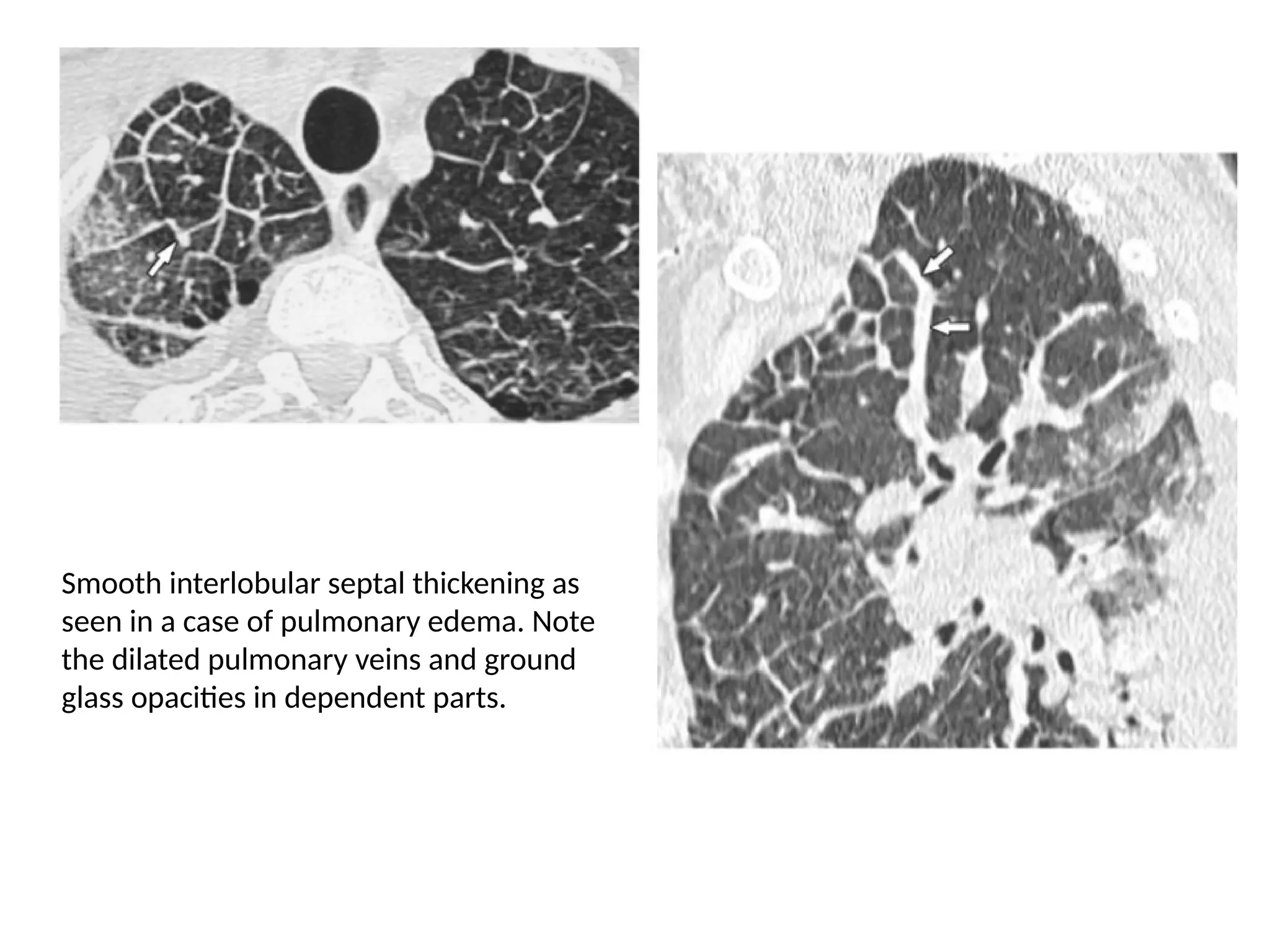
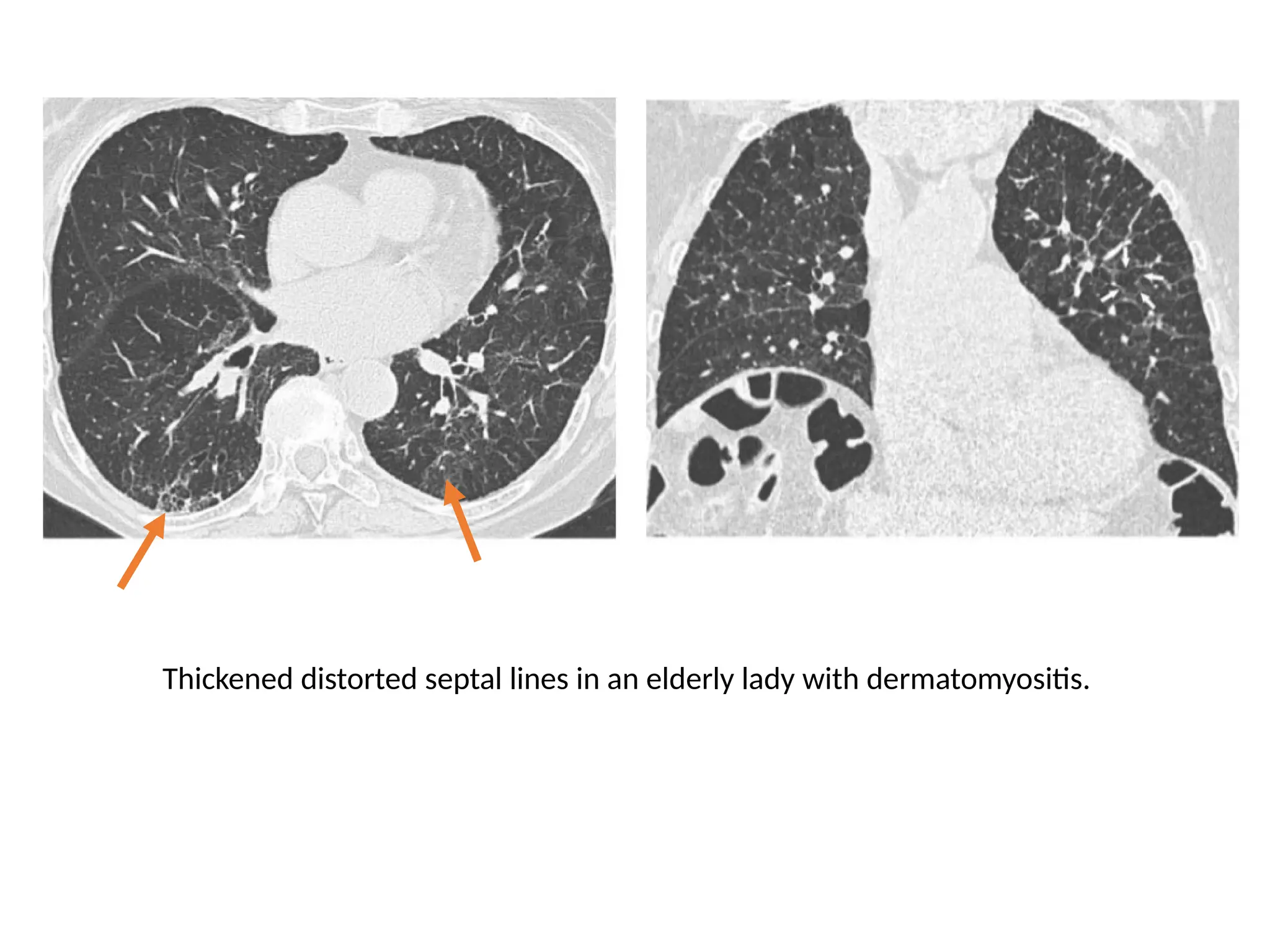
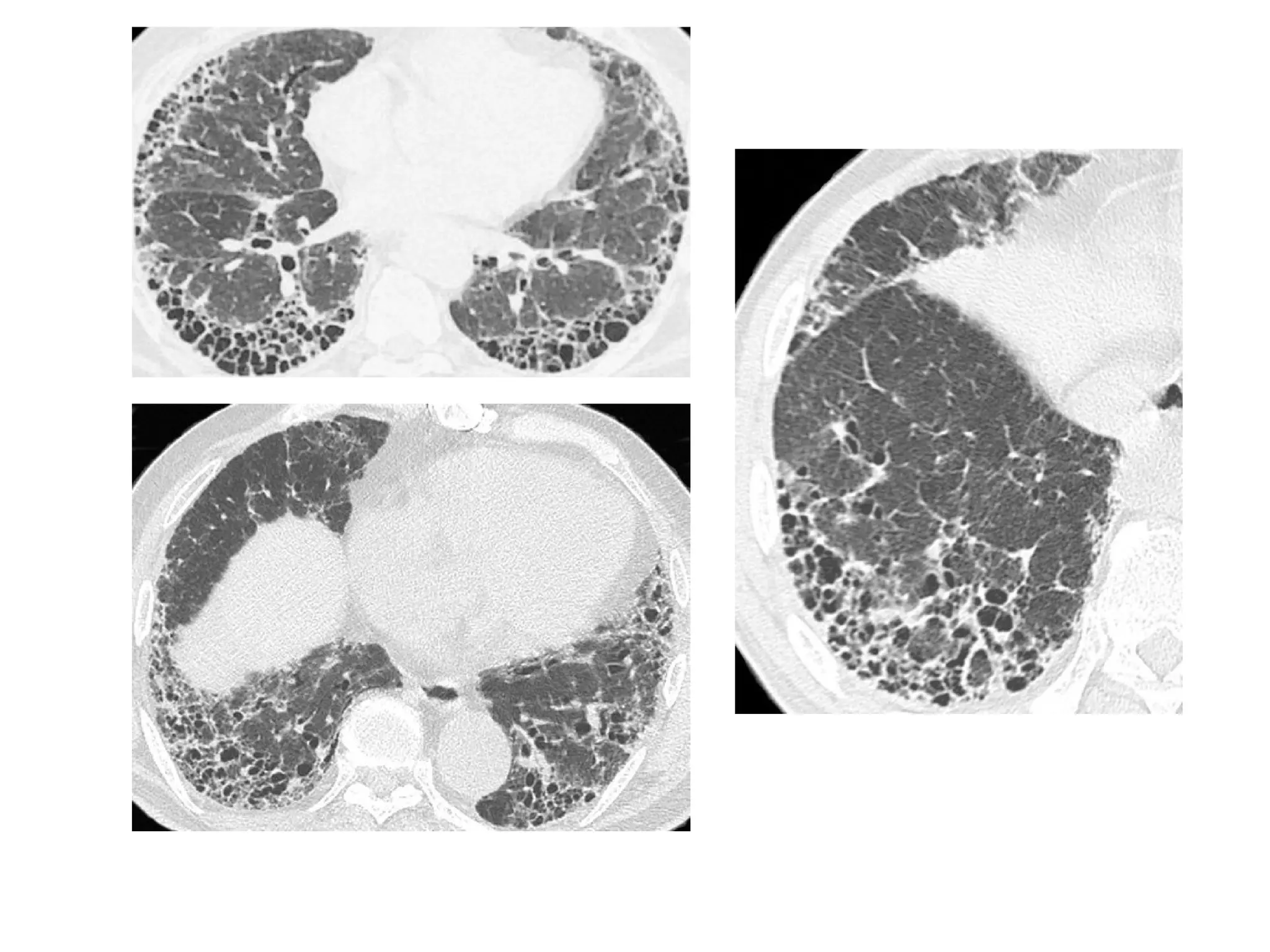
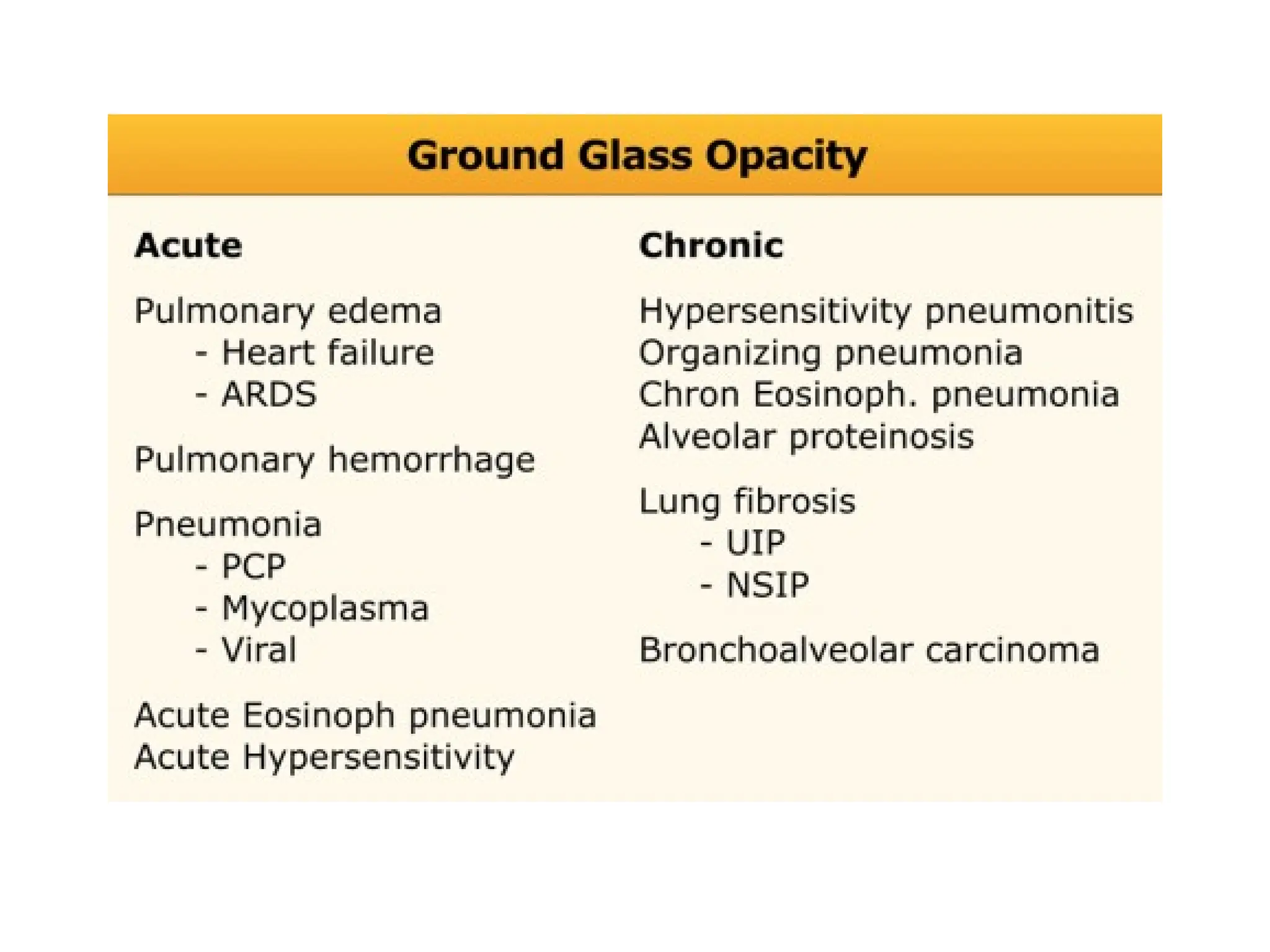
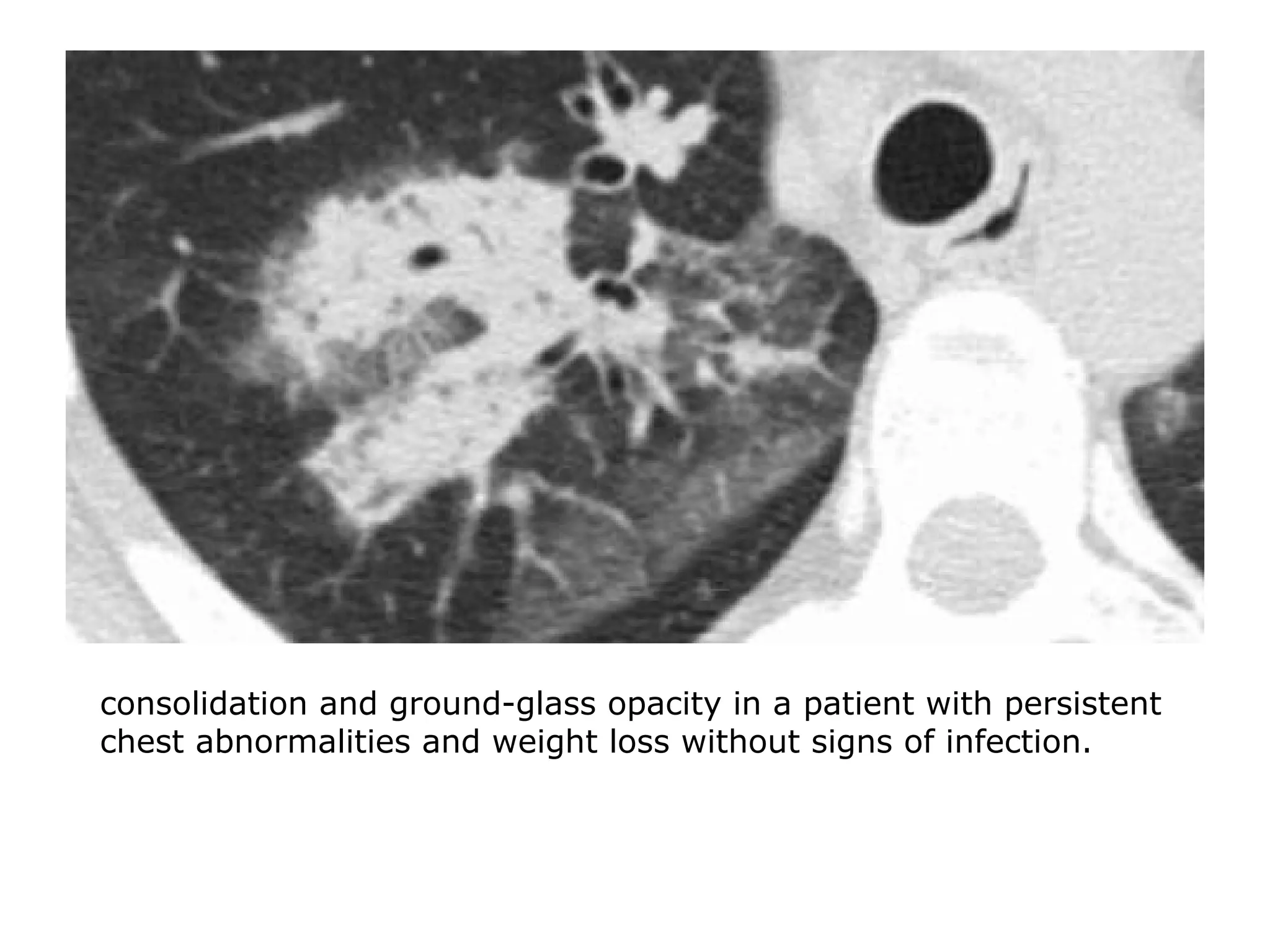
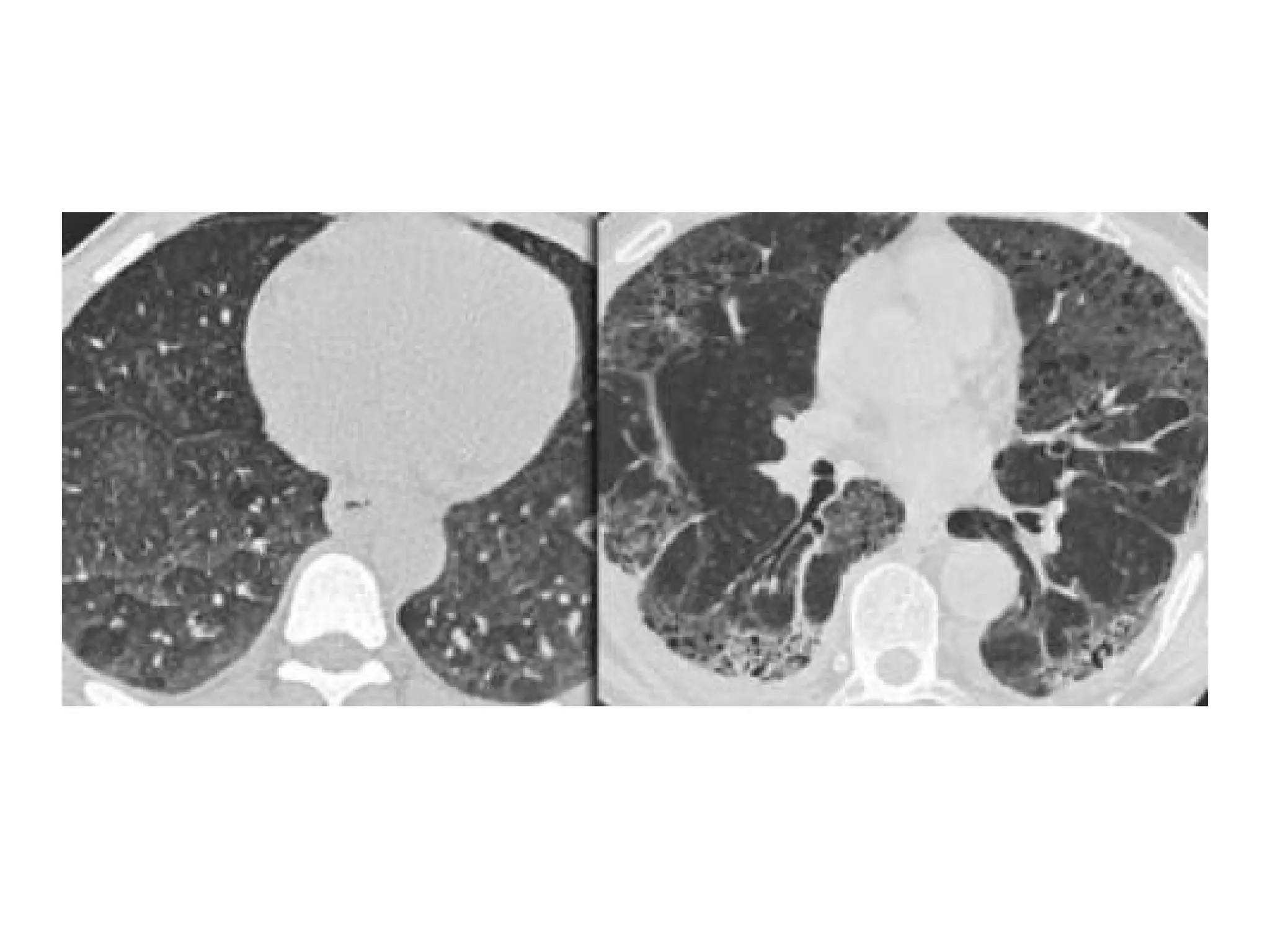
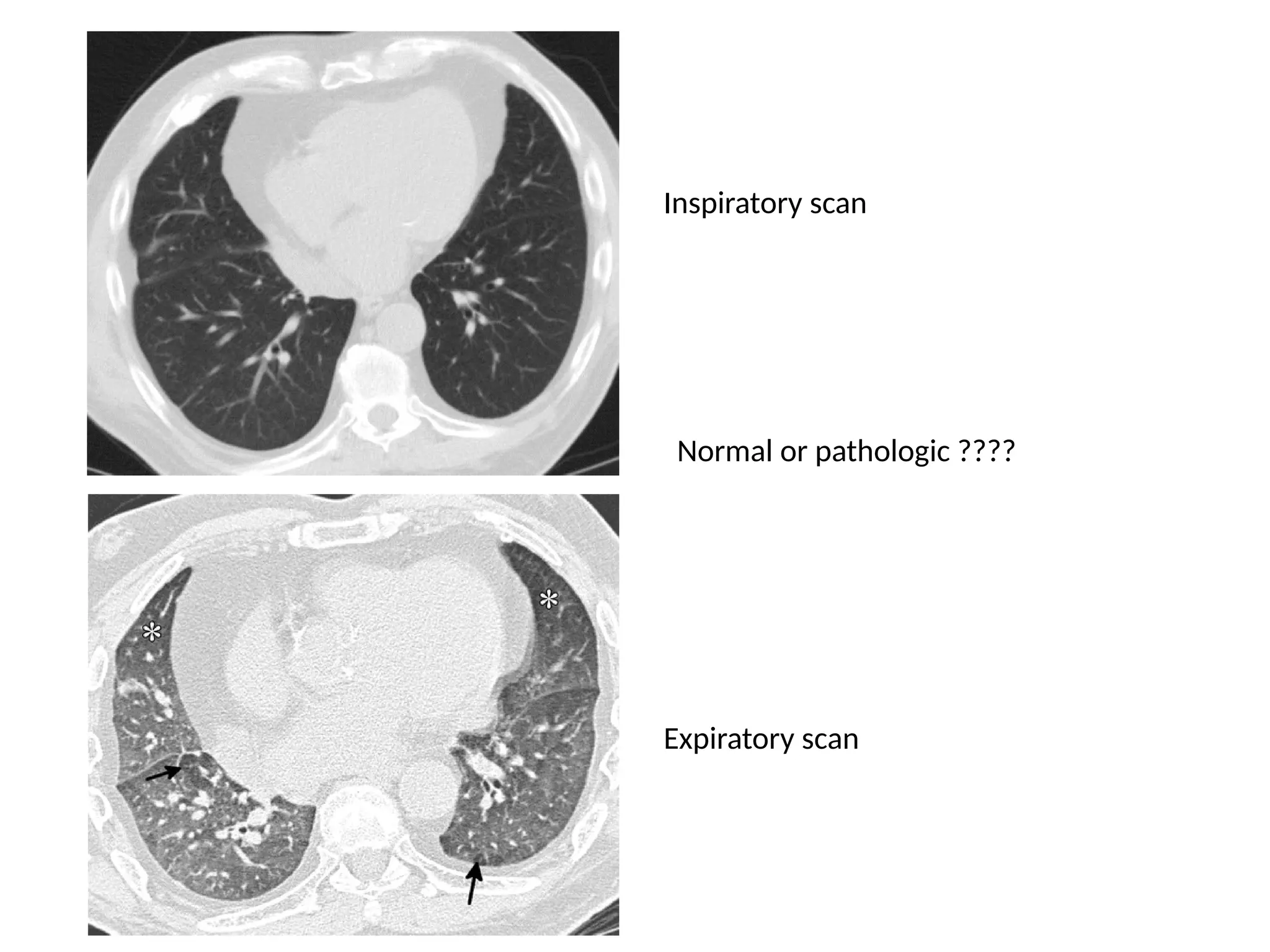
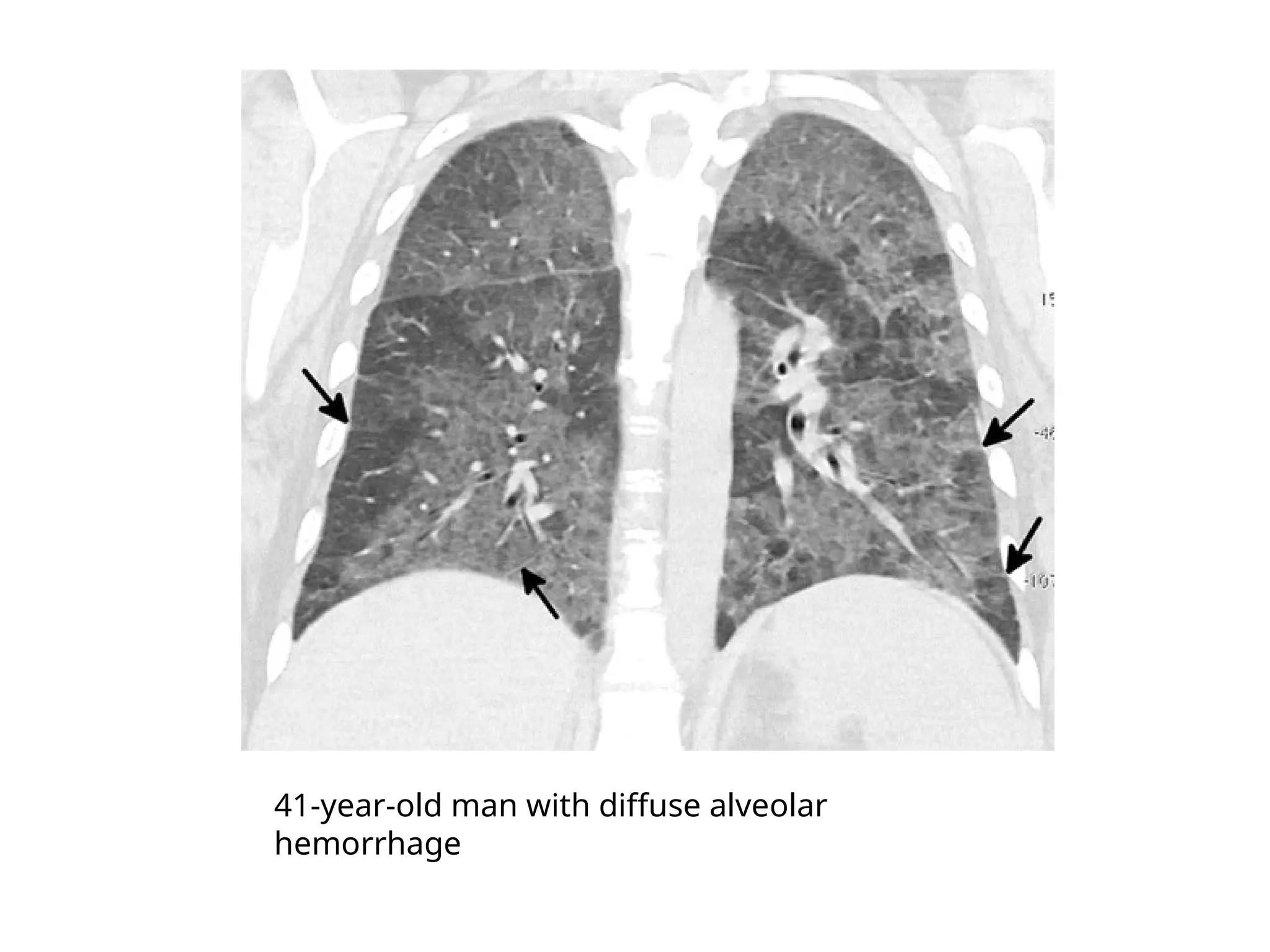
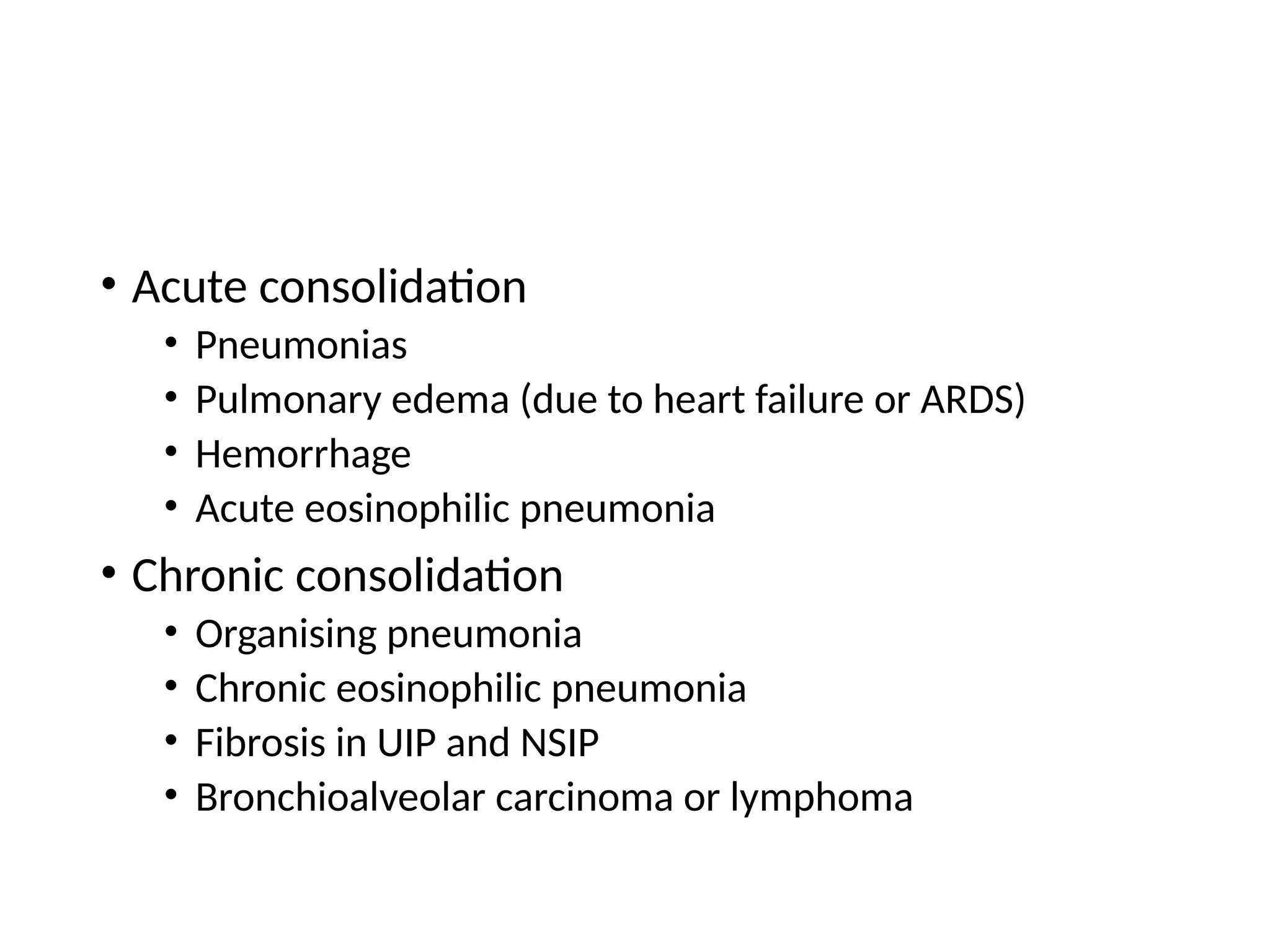
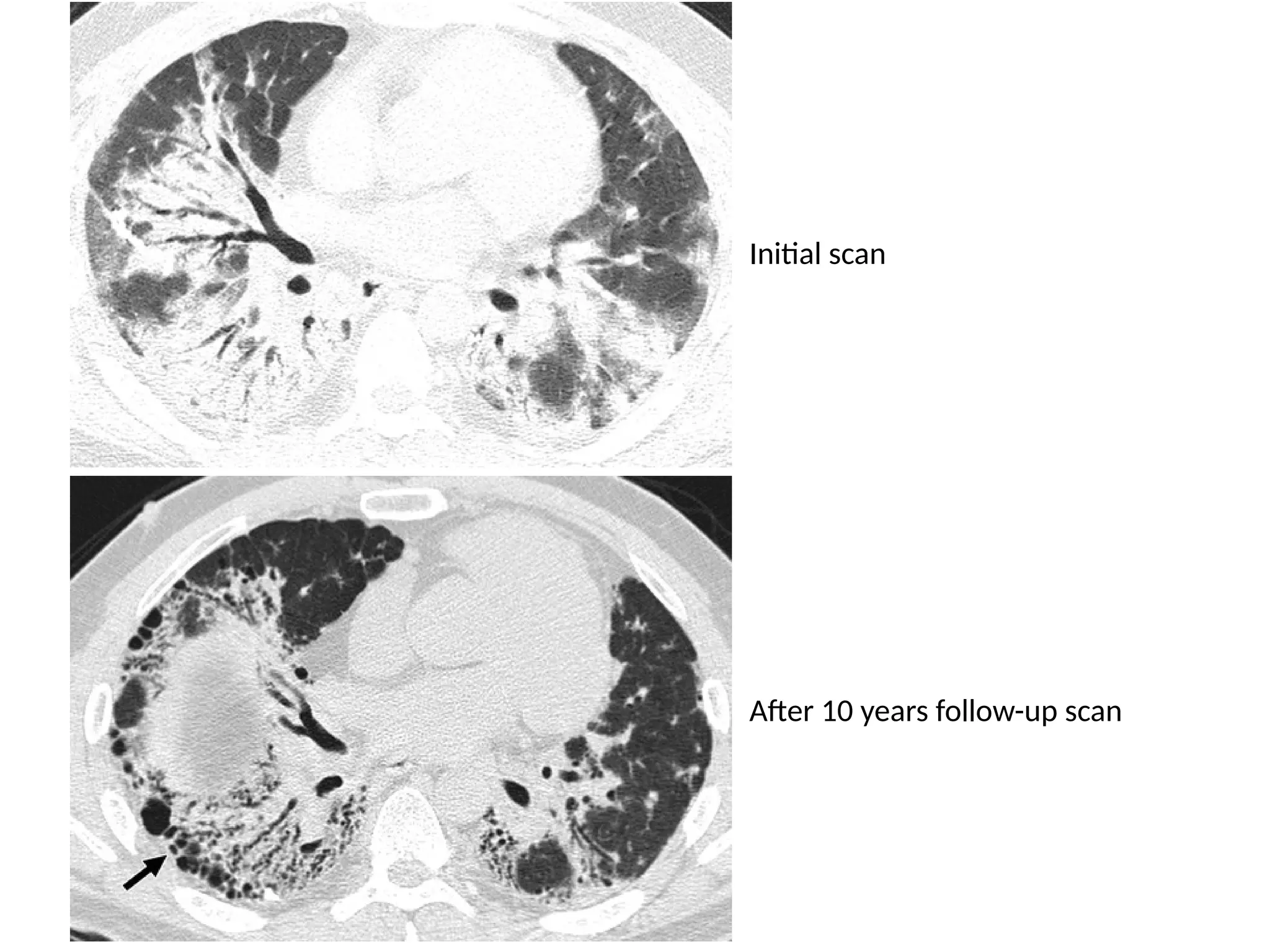
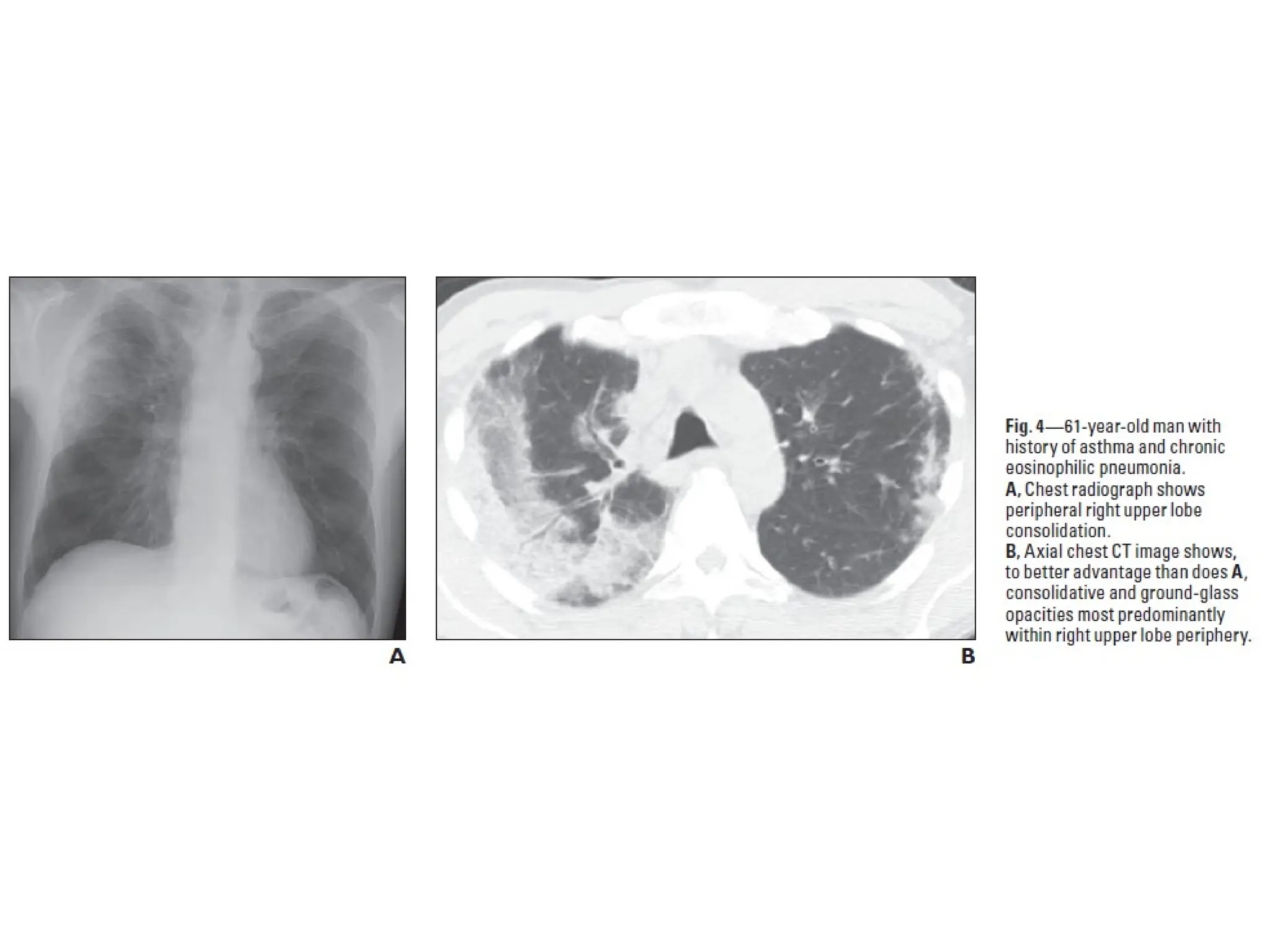
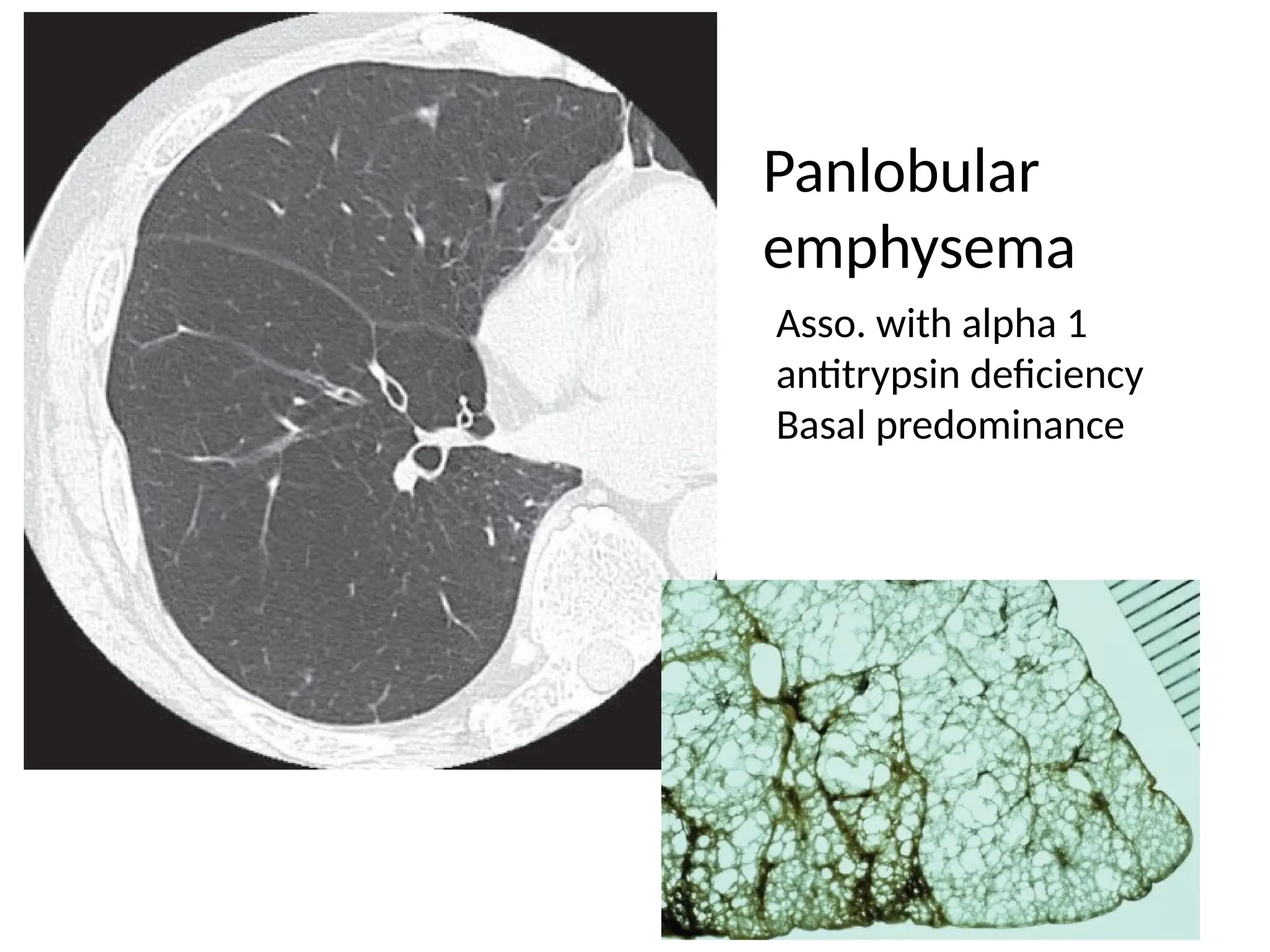
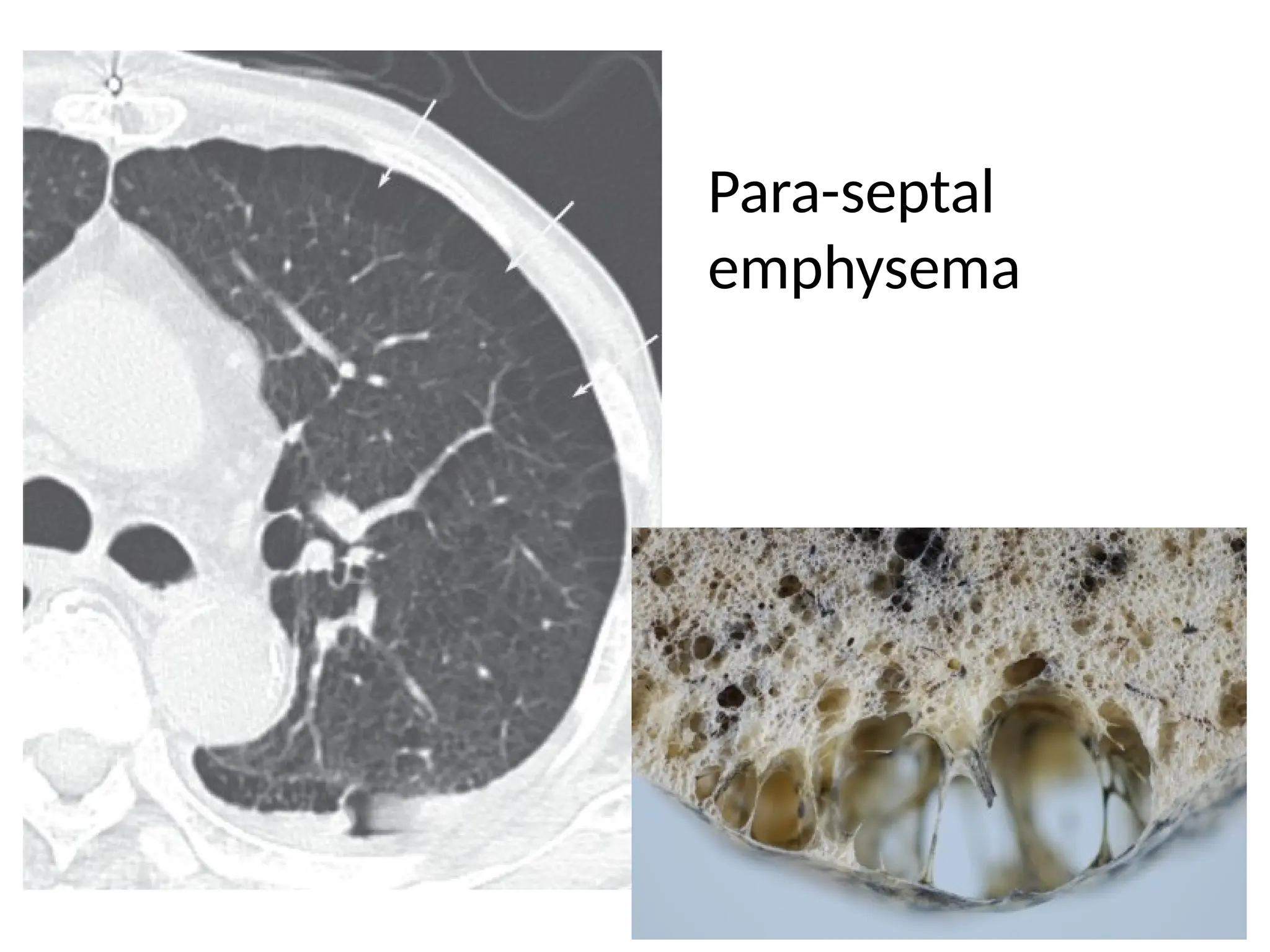
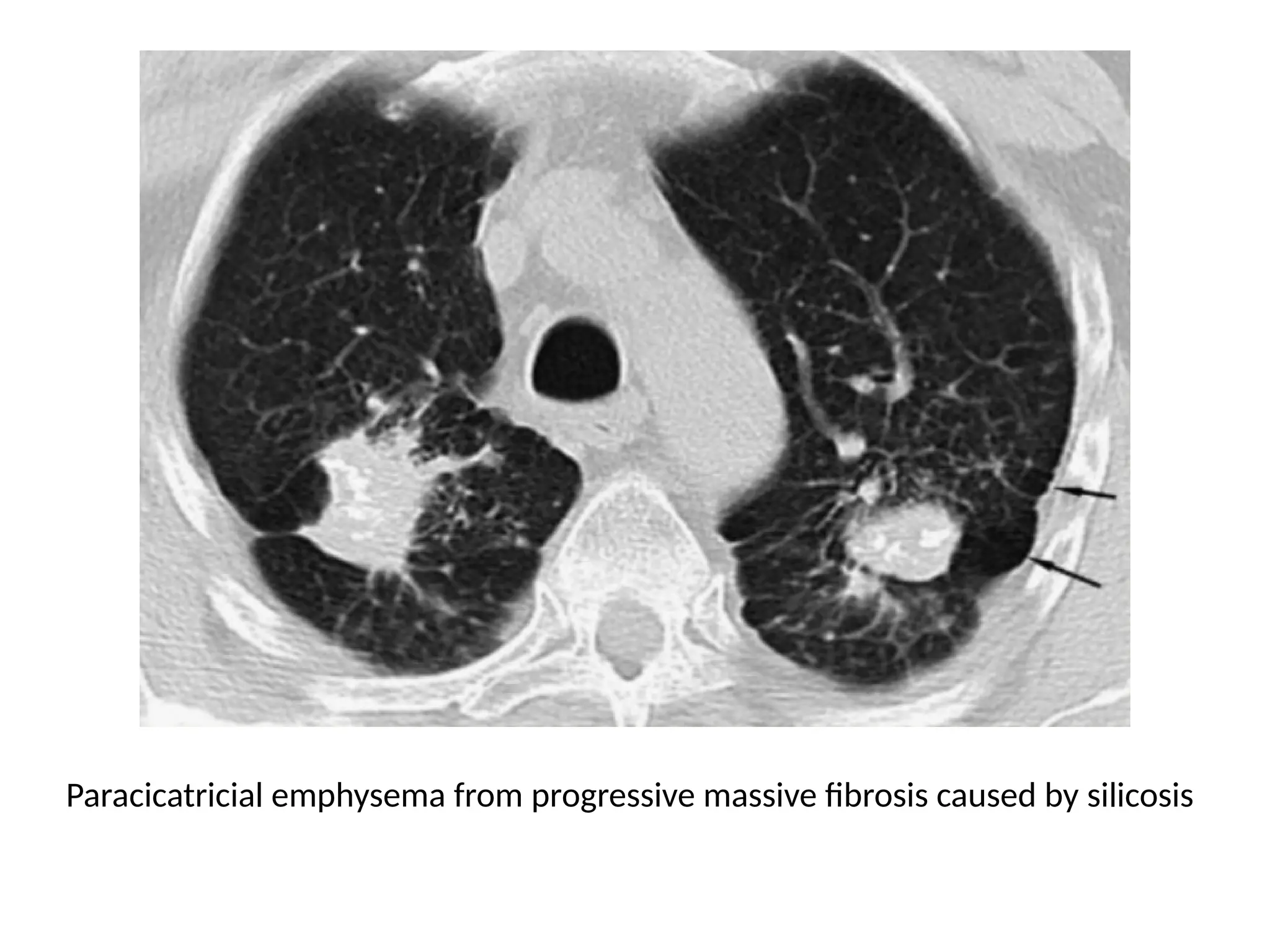
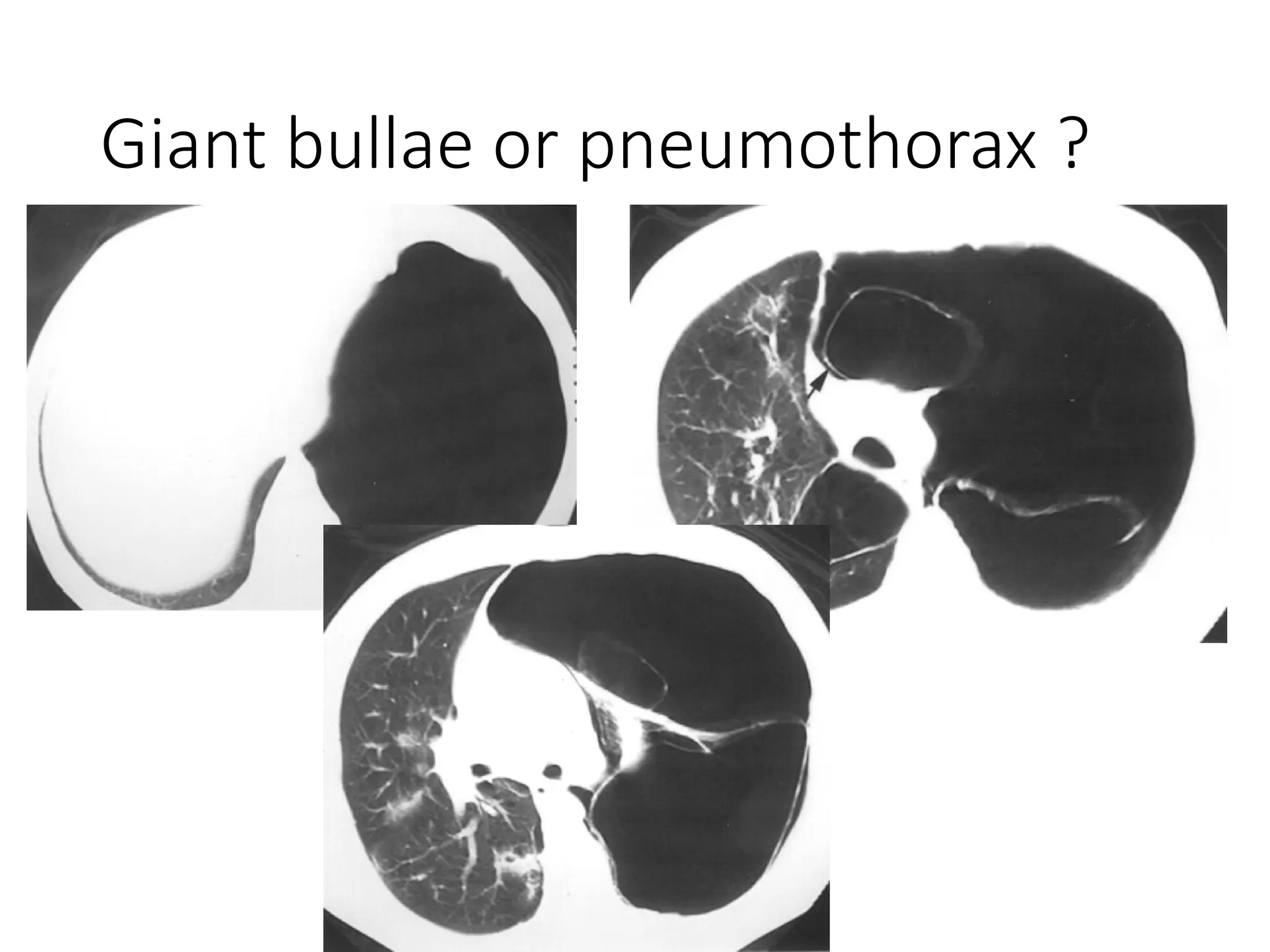
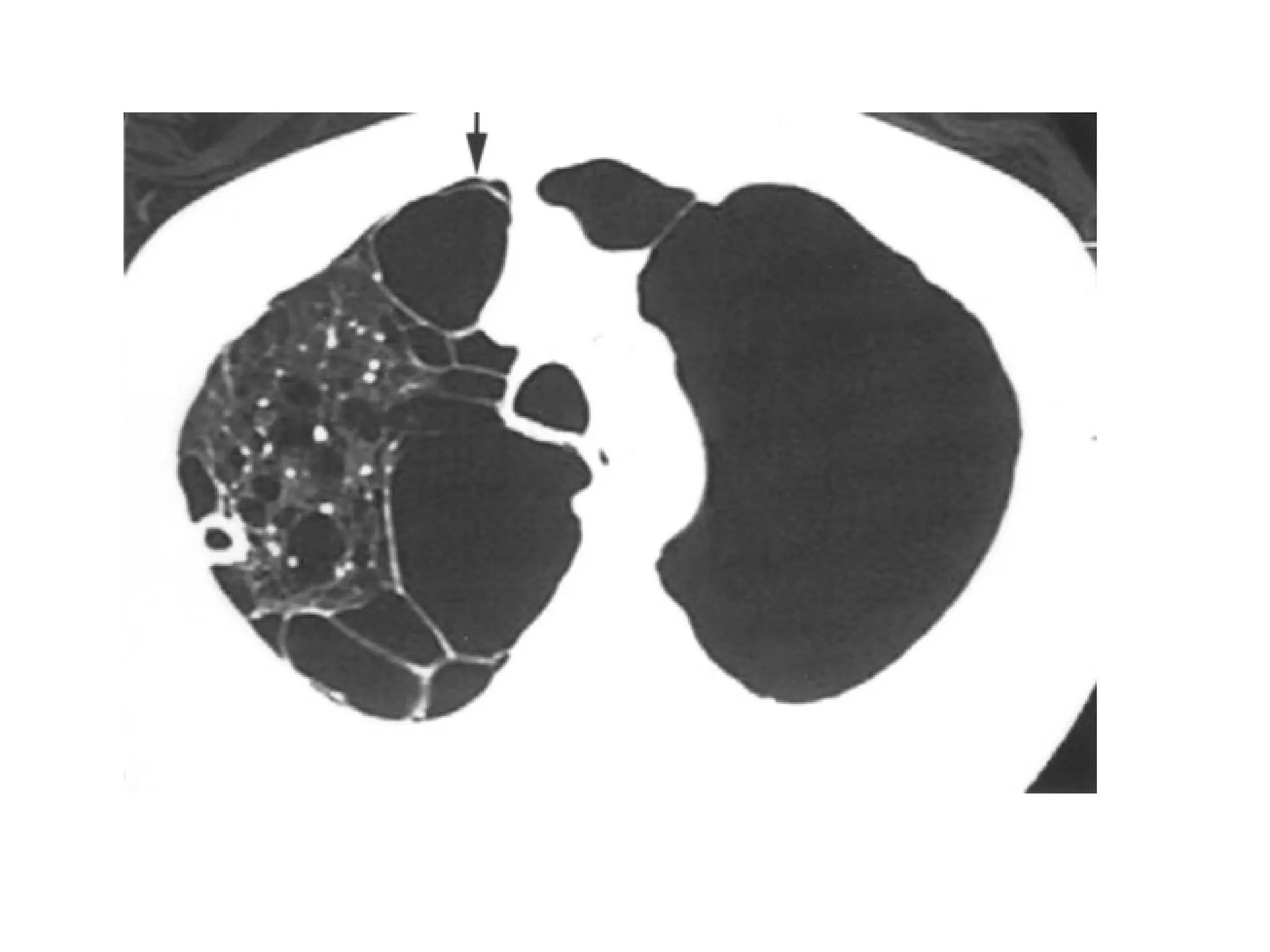
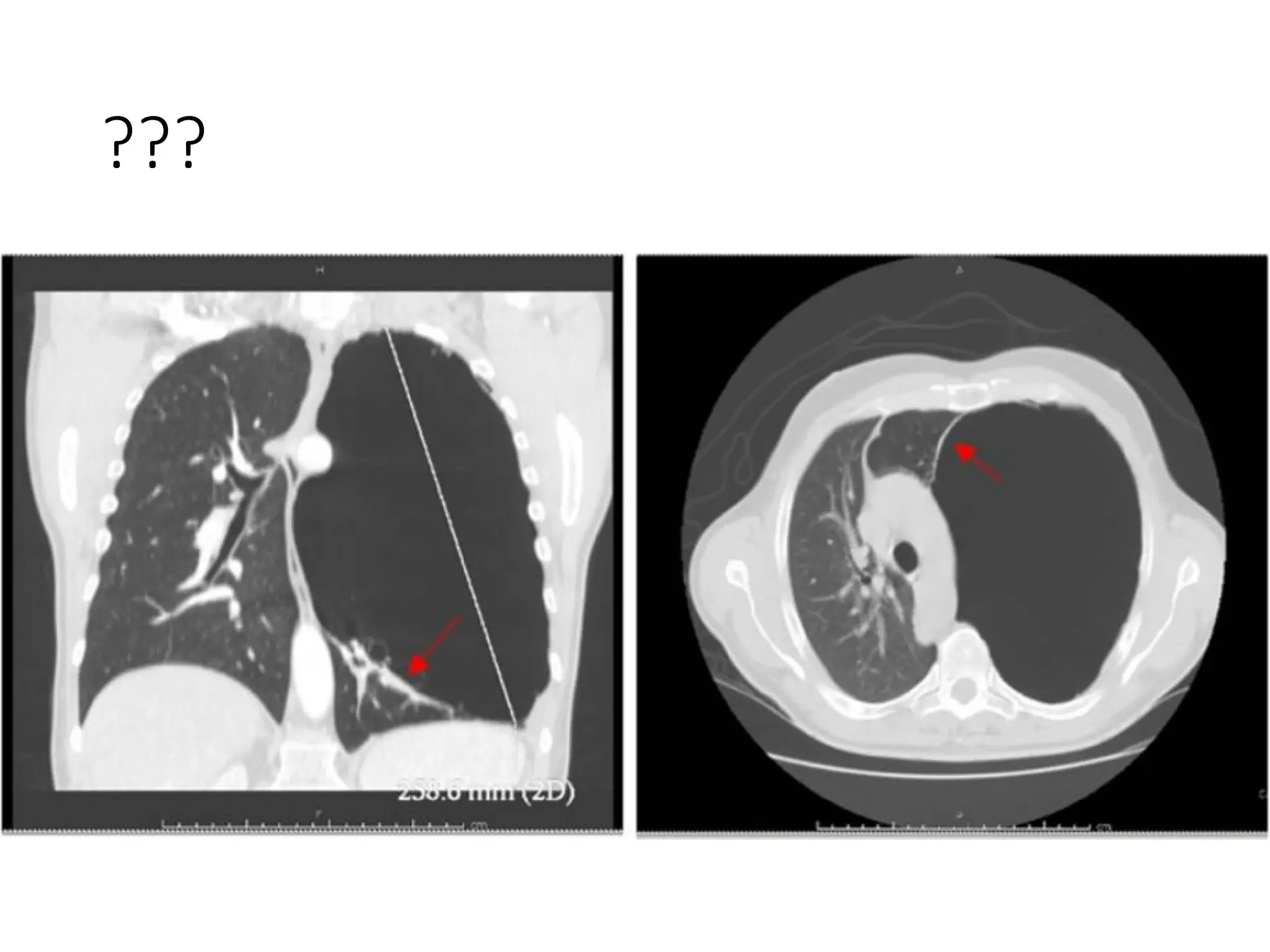
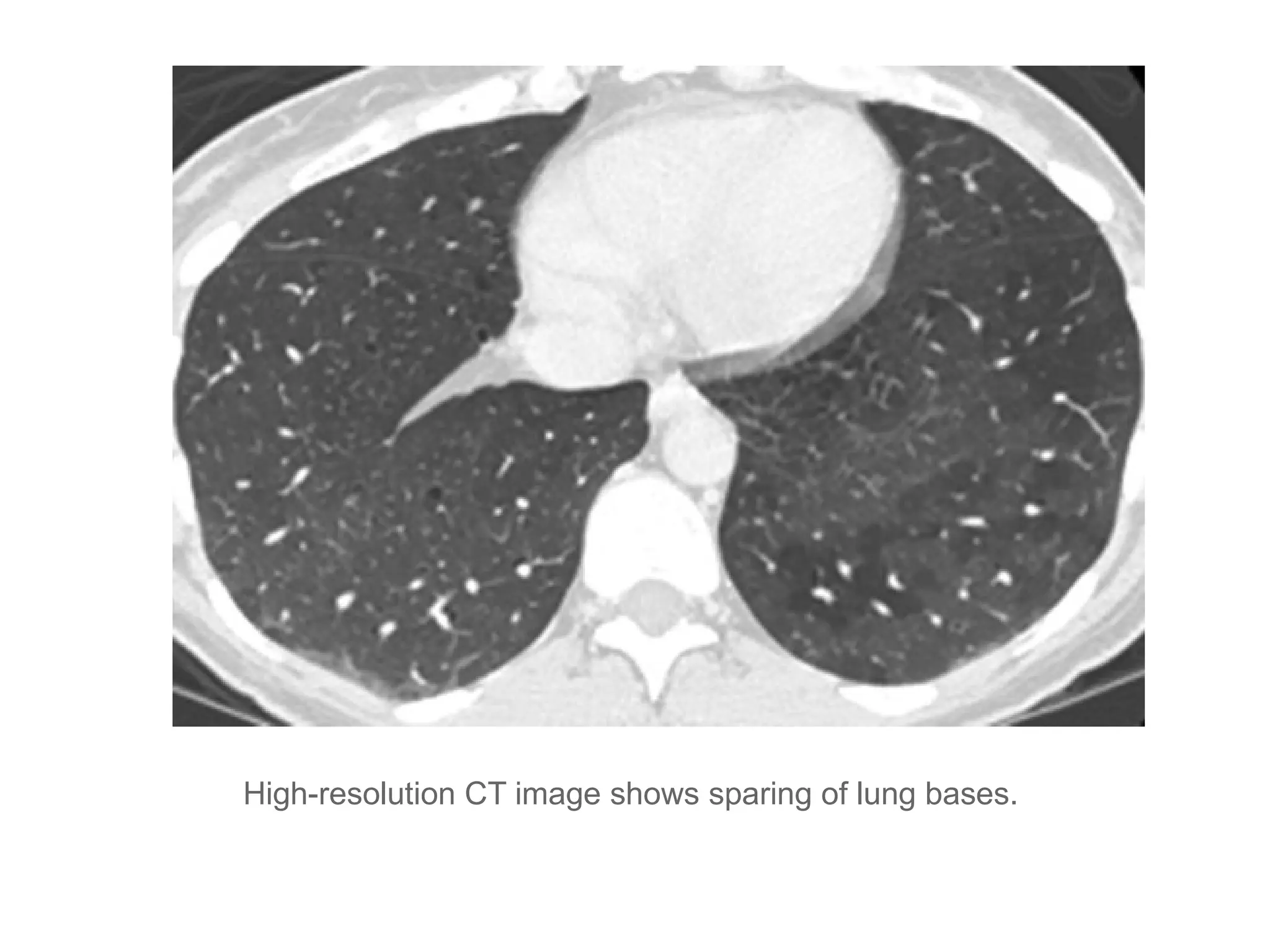
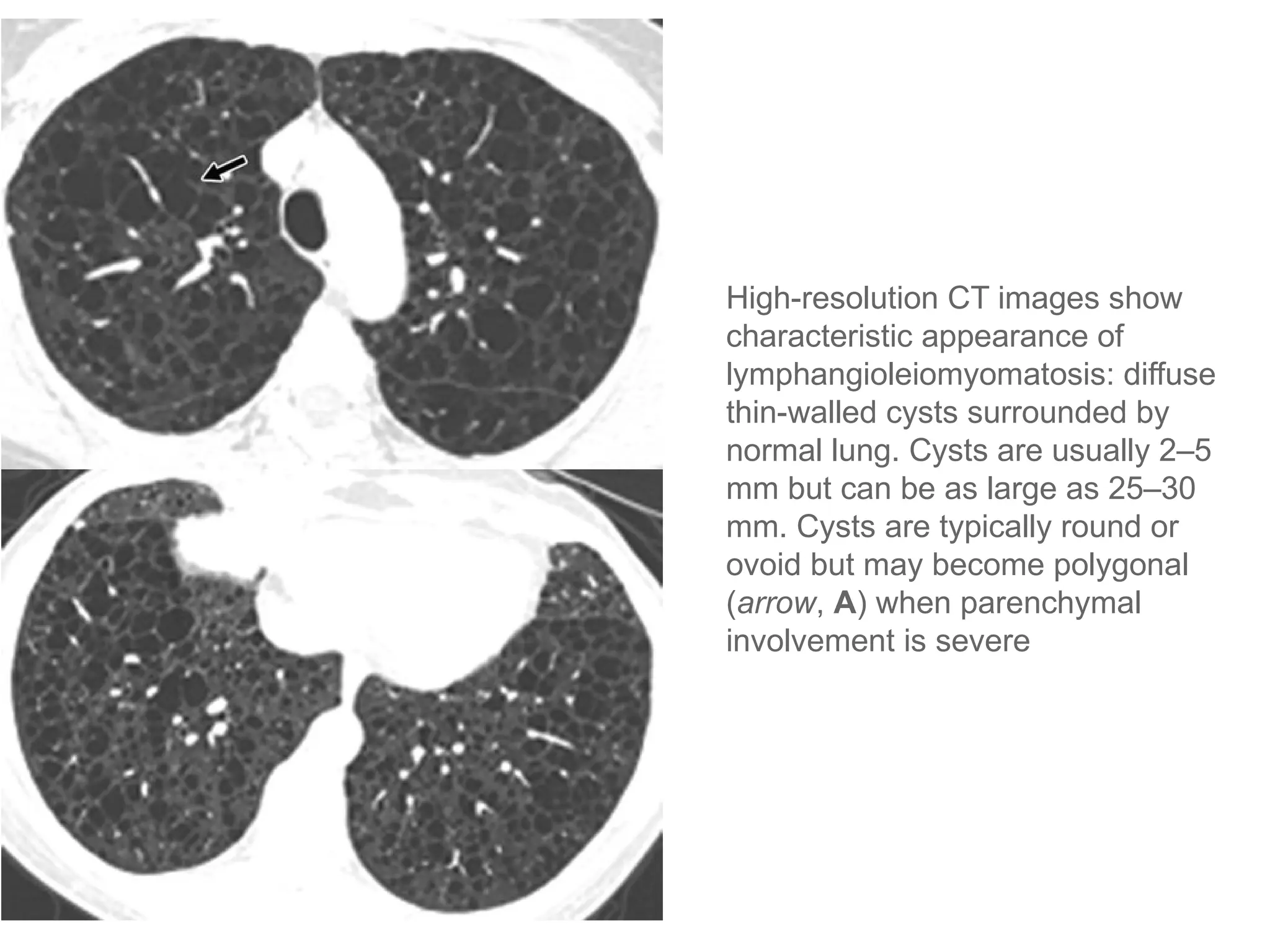
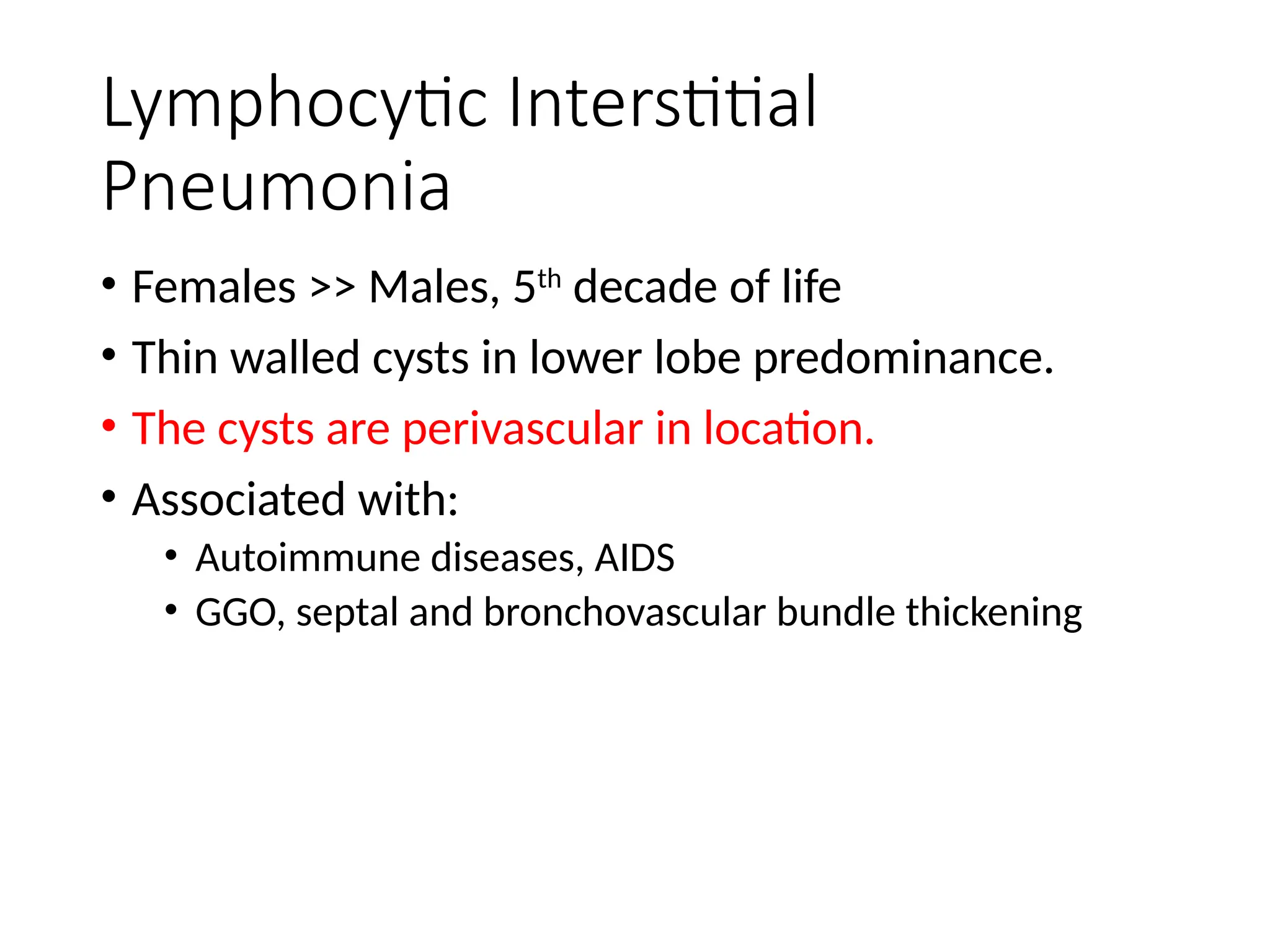
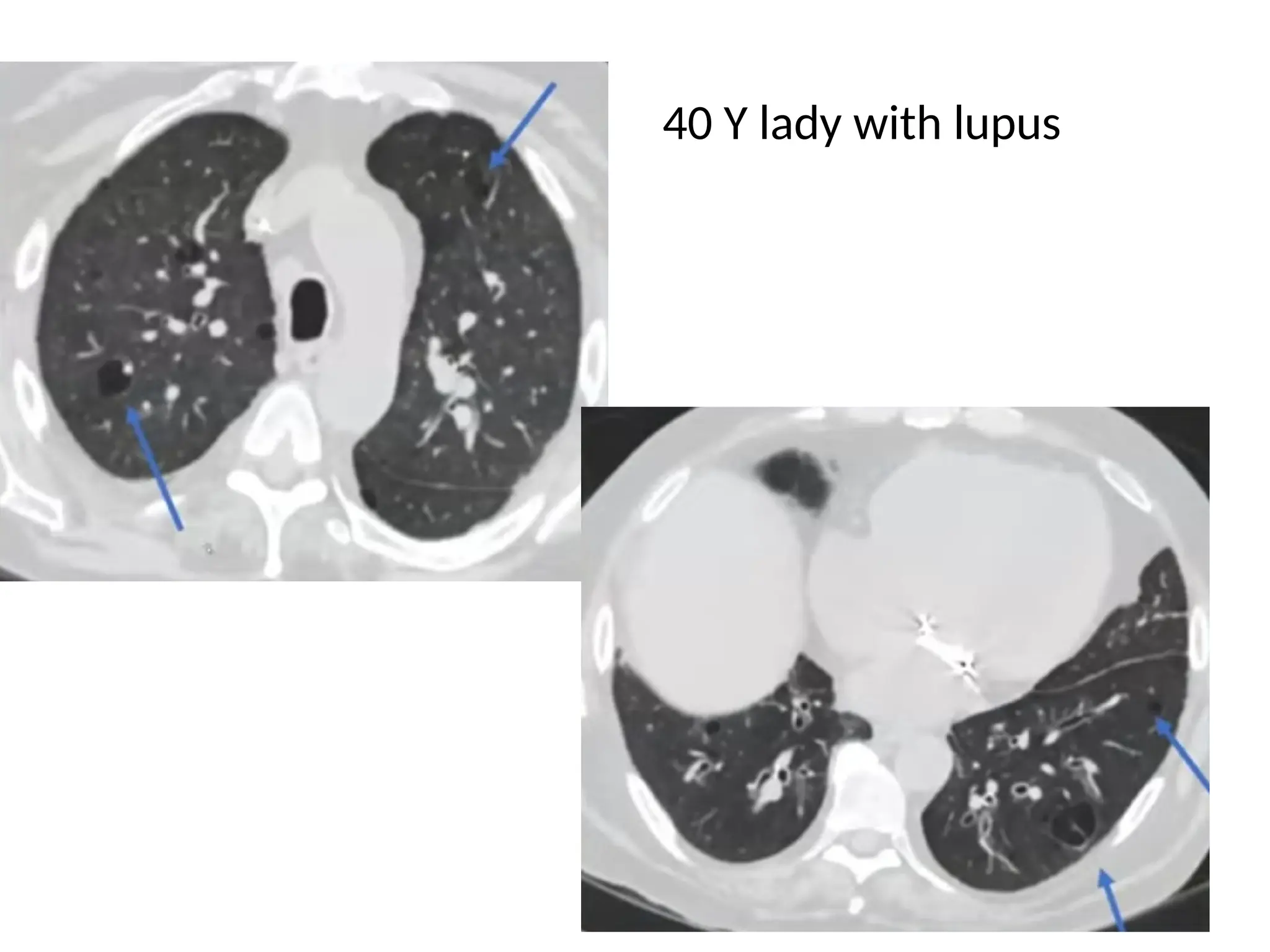
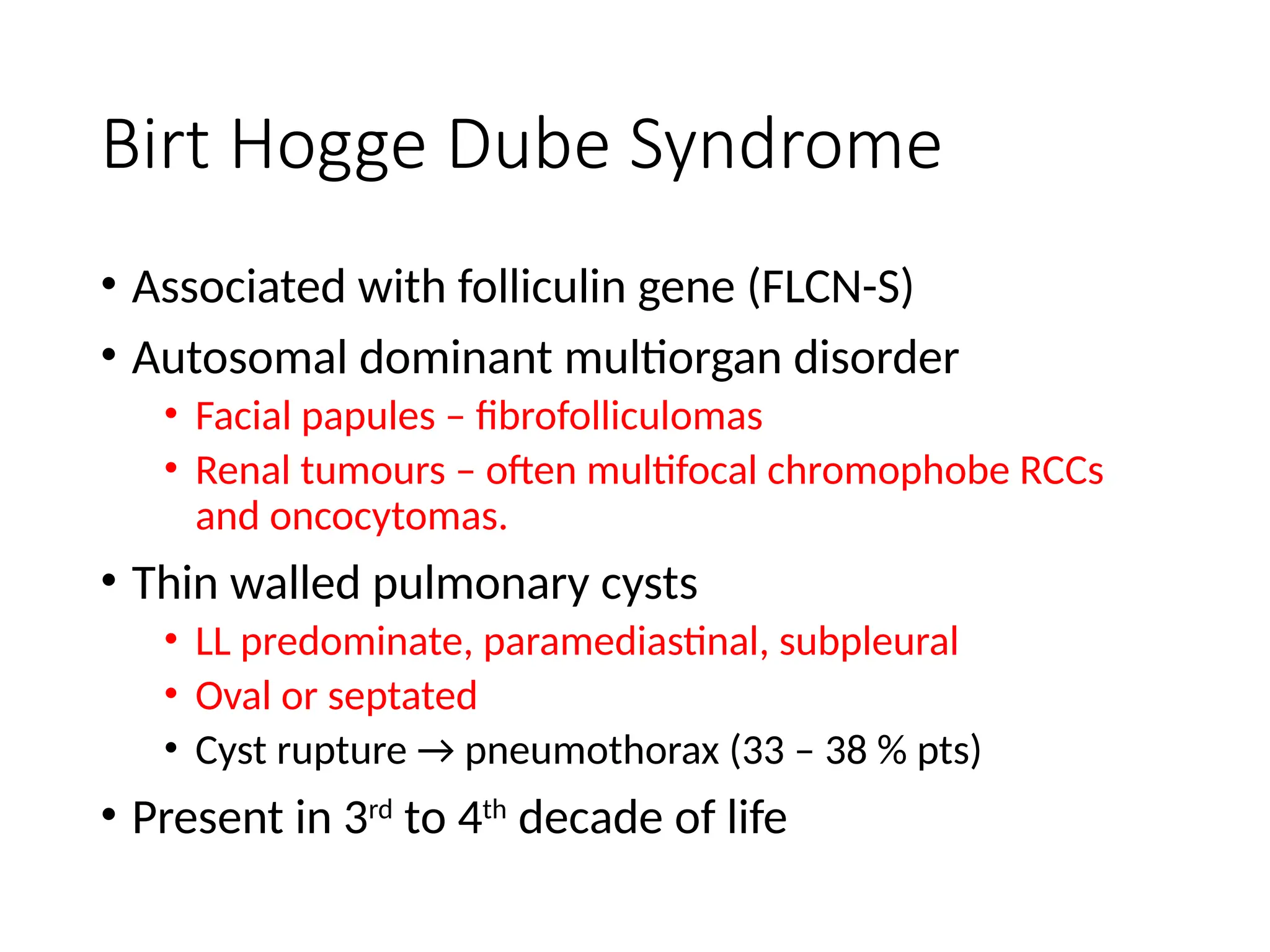
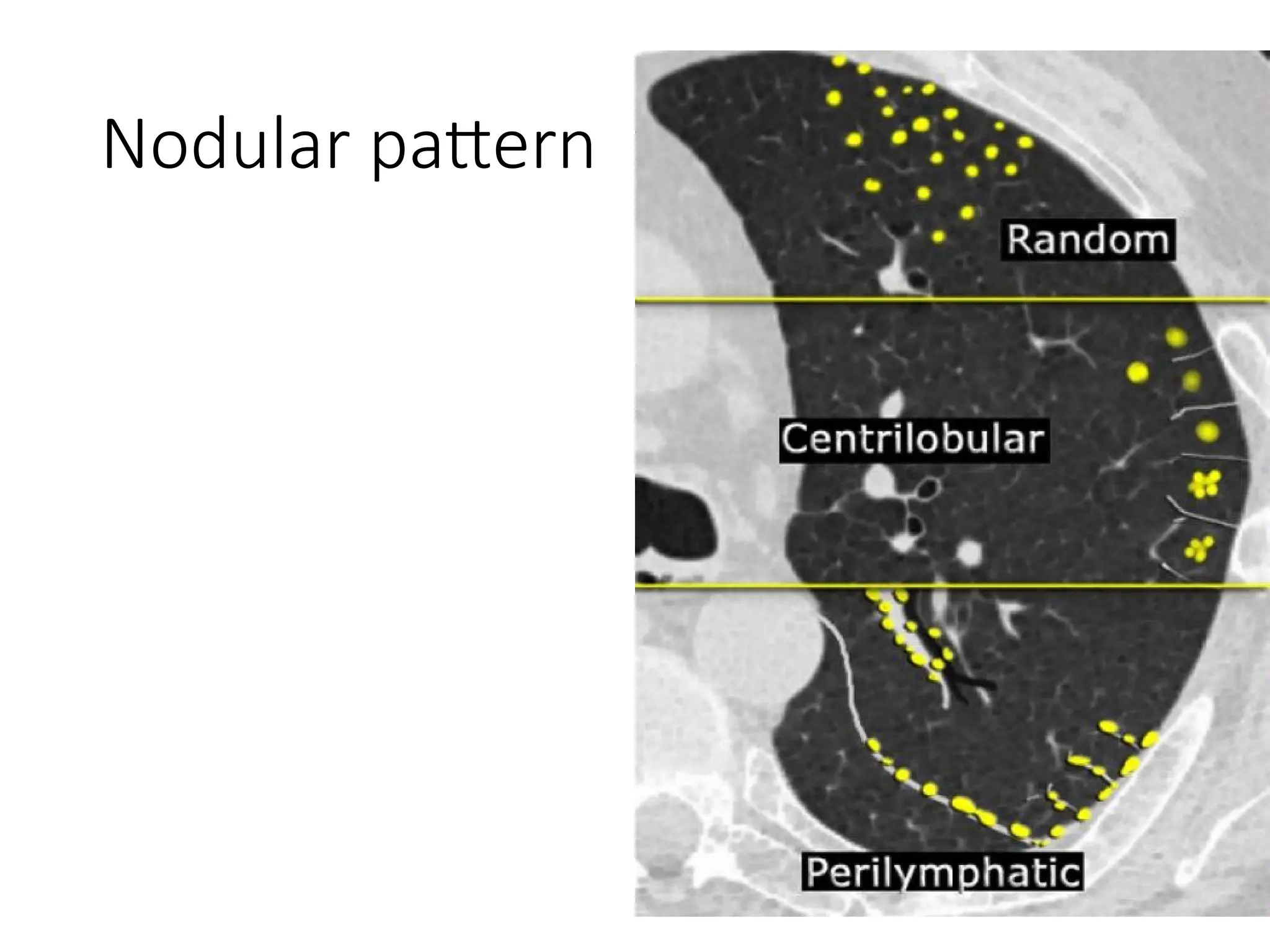
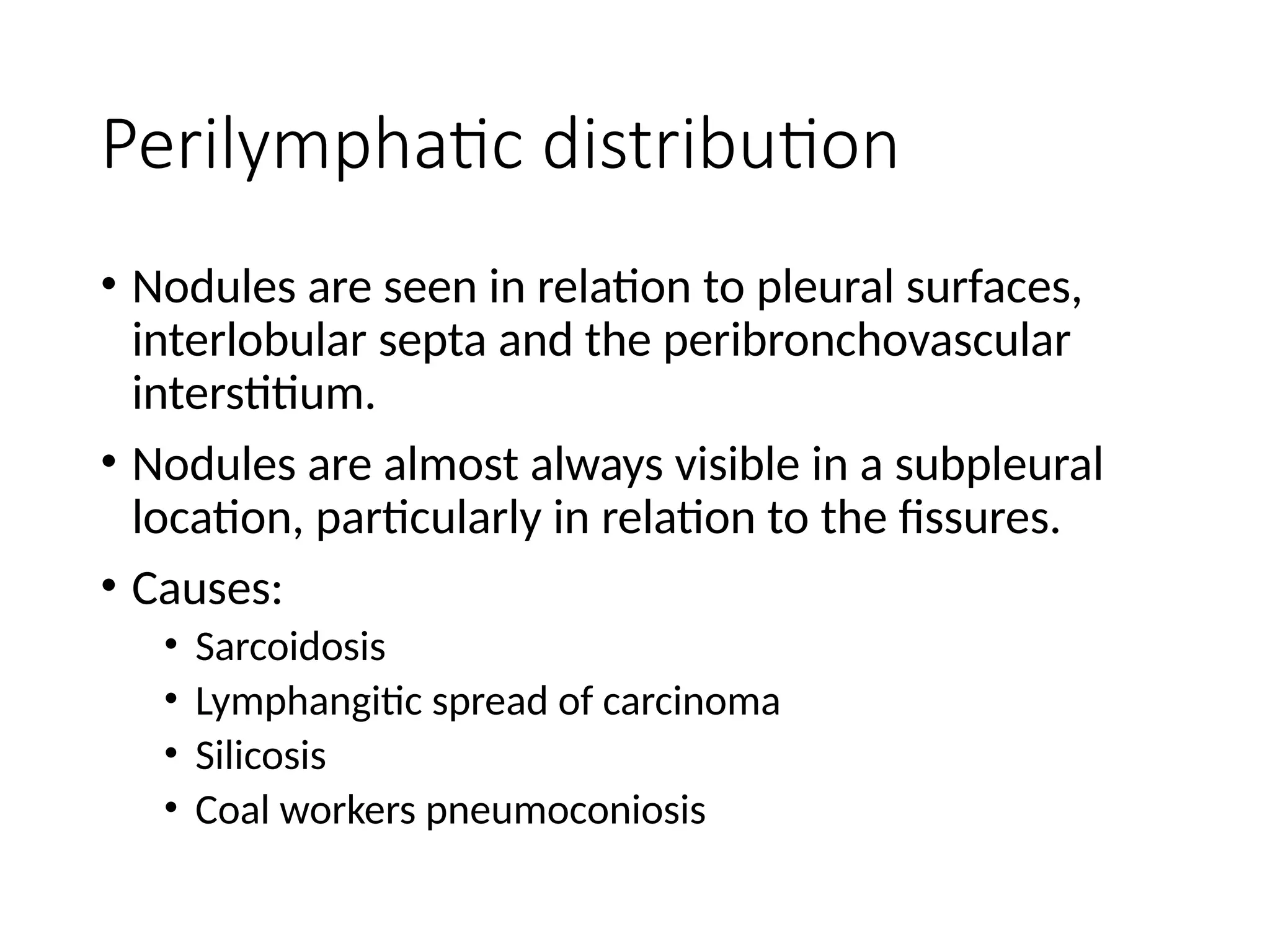
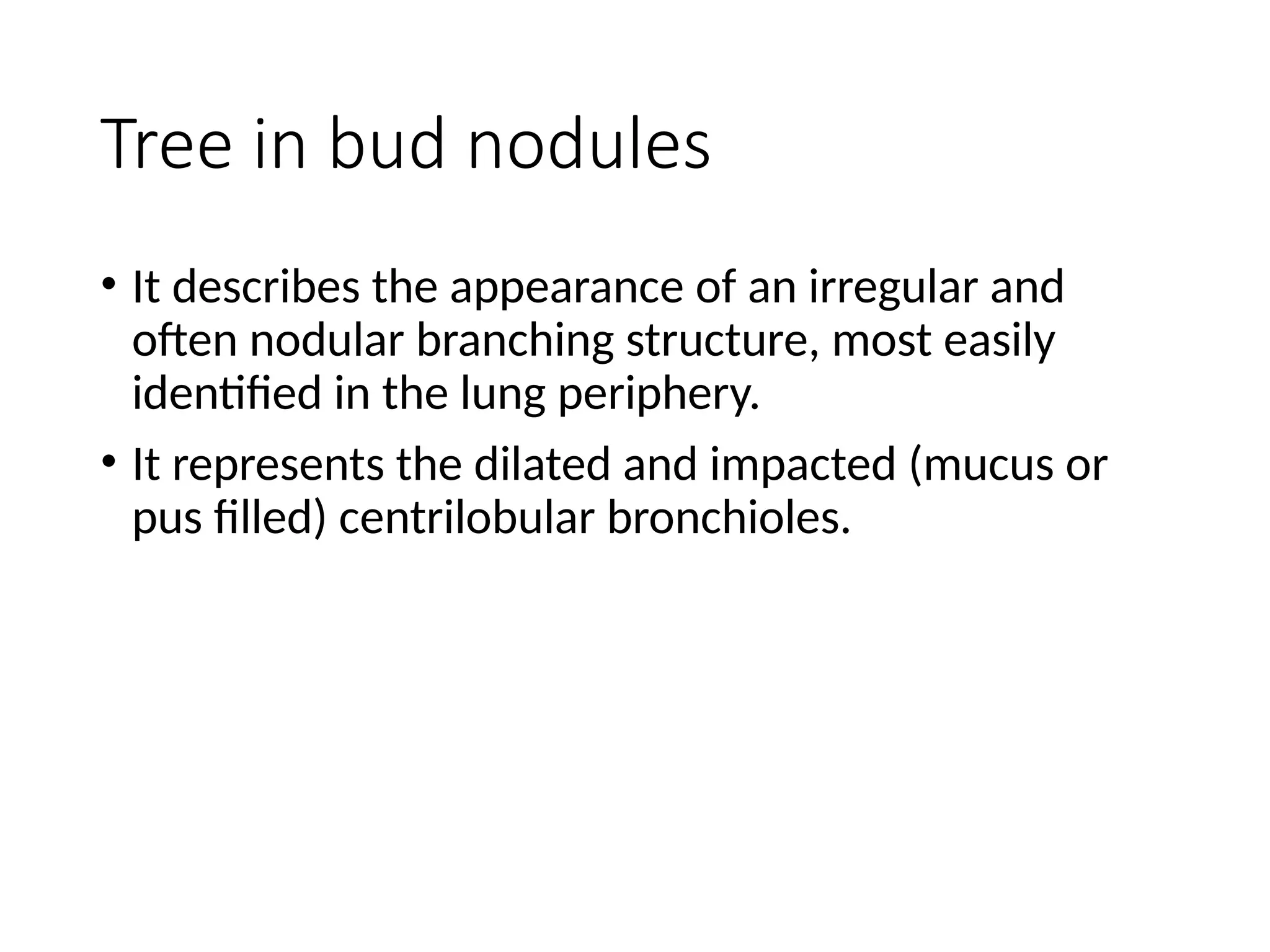
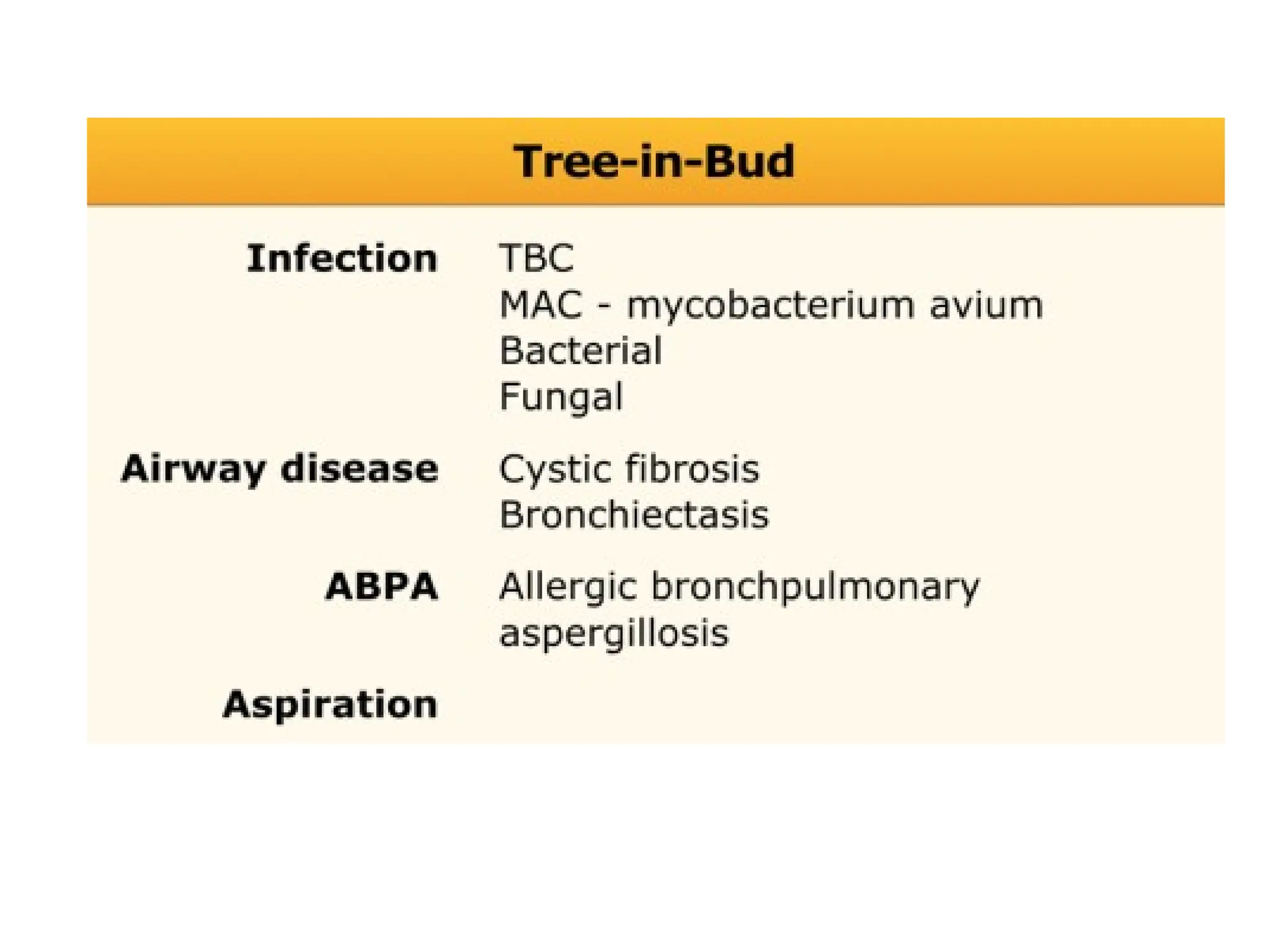
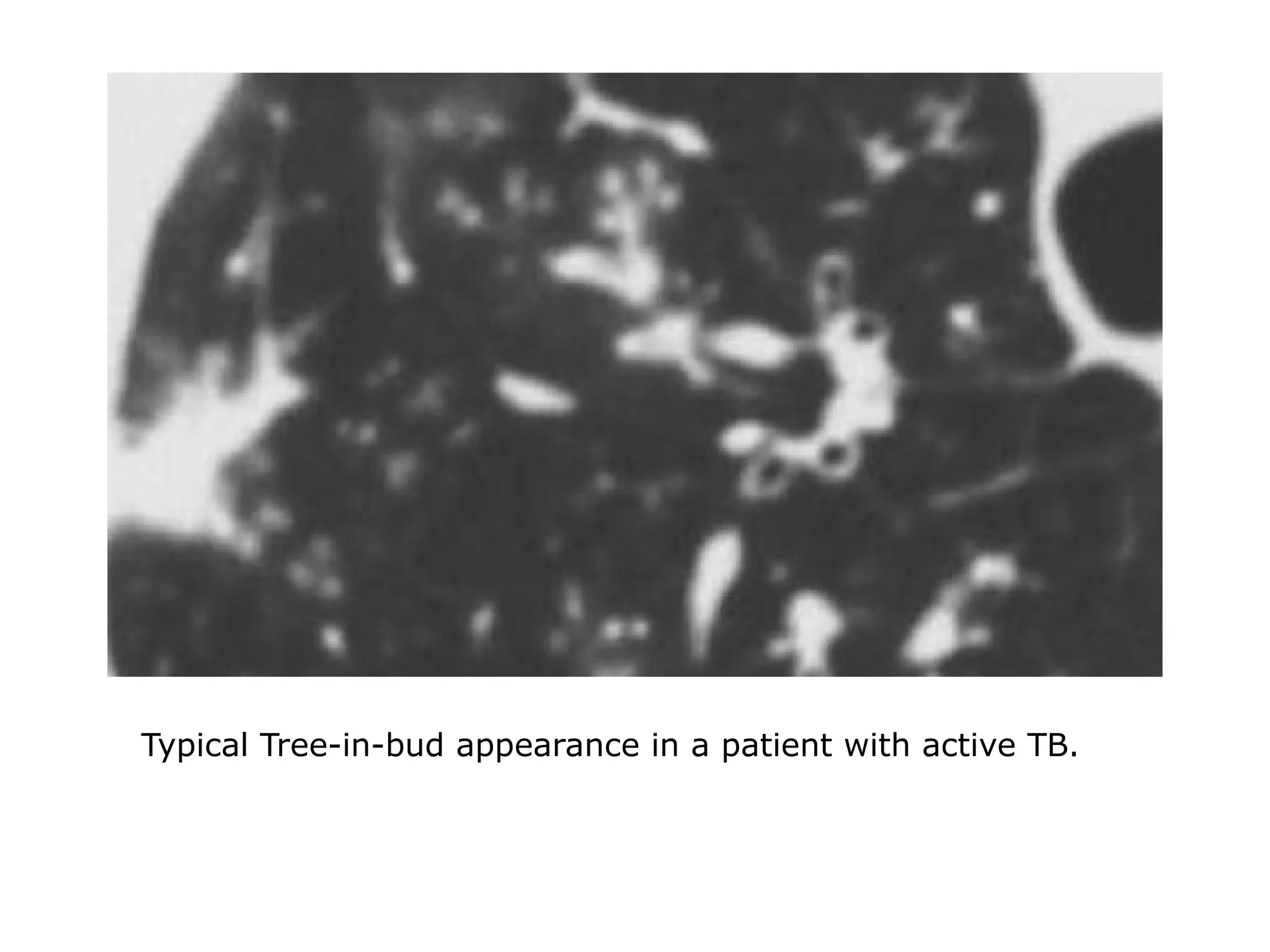
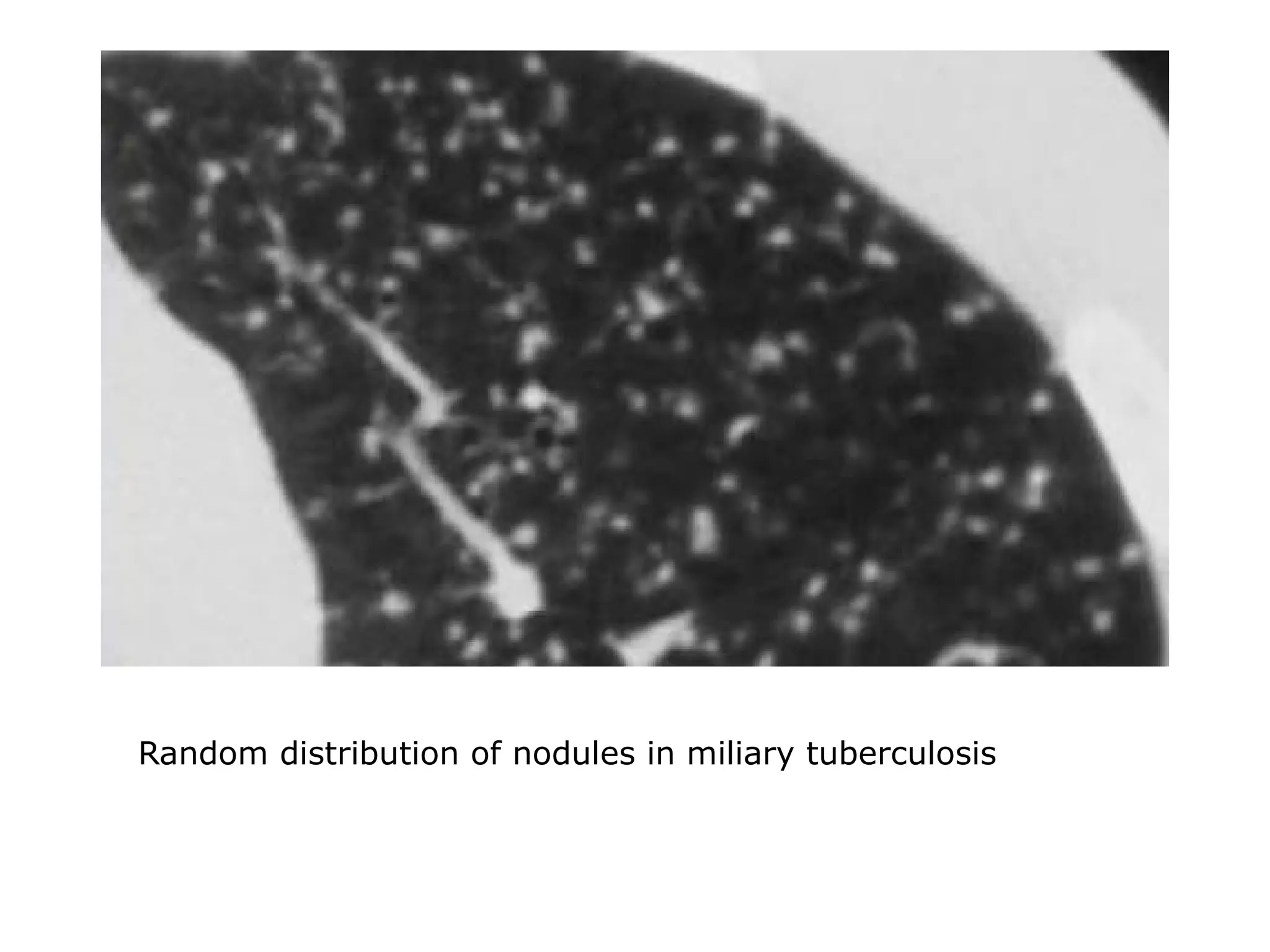
The document presents an overview of high-resolution computed tomography (HRCT) patterns in lung diseases, emphasizing the advantages of multidetector row CT (MDCT) for better visualization of lung parenchyma. It discusses various HRCT patterns such as reticular, nodular, ground-glass opacities, and consolidation, along with specific conditions like Langerhans cell histiocytosis and non-specific interstitial pneumonitis, including their imaging characteristics and clinical significance. The document also details diagnostic approaches, imaging protocols, and key anatomical features relevant to HRCT interpretation in the context of lung pathology.