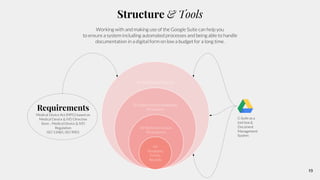
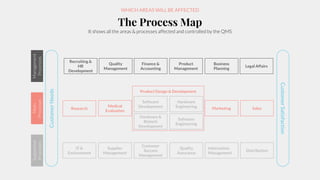
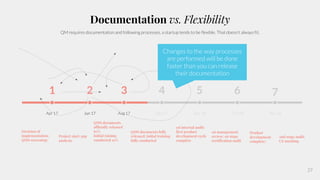
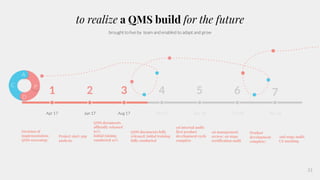
The document outlines the implementation of a Quality Management System (QMS) based on ISO 9001:2015 and ISO 13485:2016, emphasizing the importance of customer focus, continuous improvement, and employee engagement. It provides a structured approach for startups, including key principles, process mapping, and an implementation plan that combines documentation and training. The objective is to create a flexible and effective system that enhances product development and meets regulatory requirements while fostering a culture of quality within the organization.