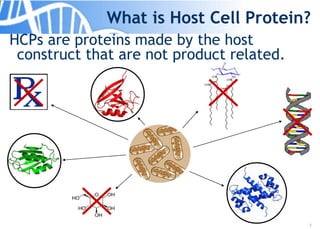
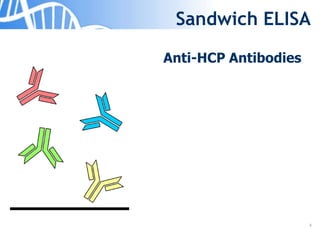
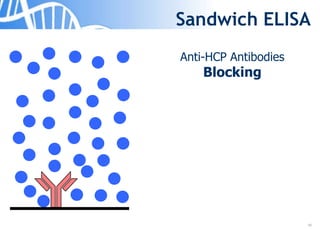
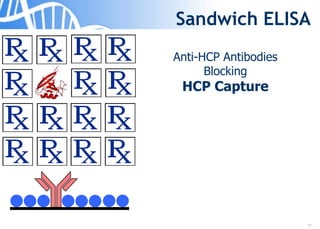
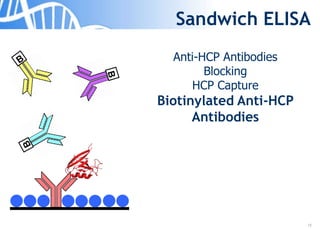
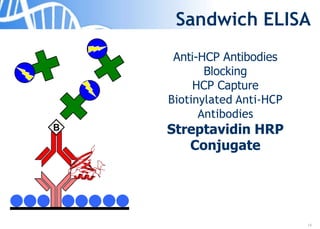
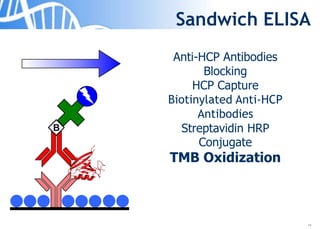
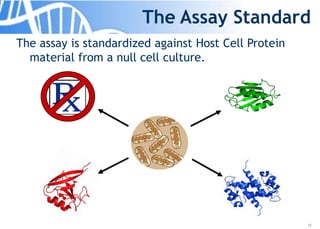
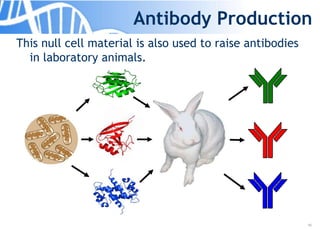
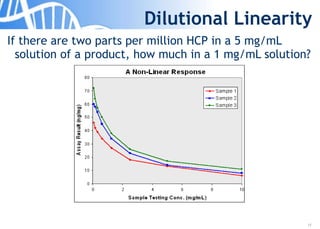
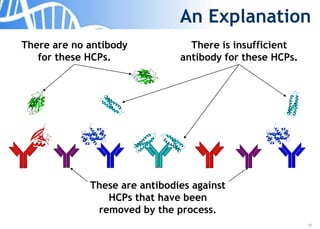
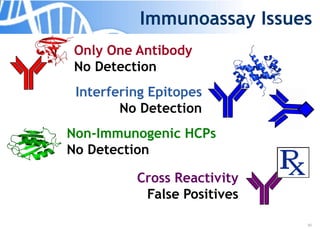
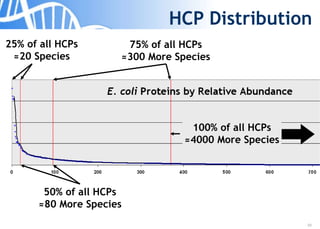
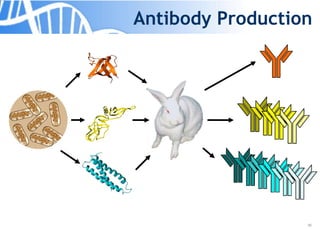
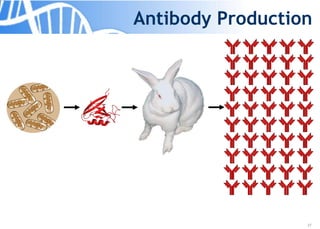
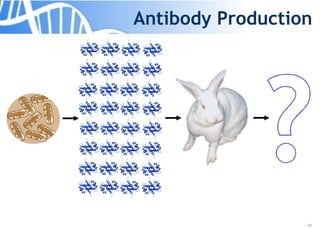
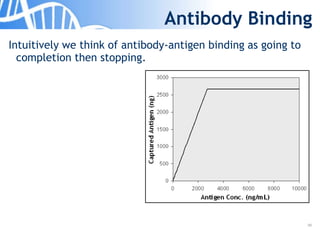
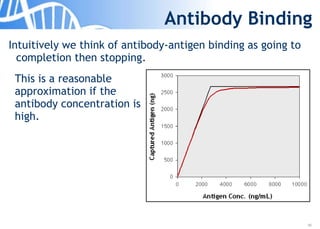
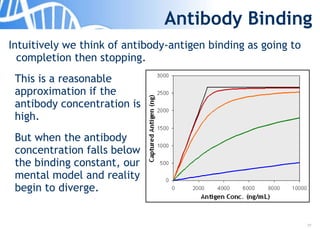
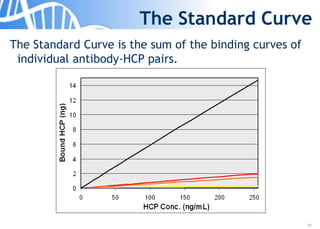
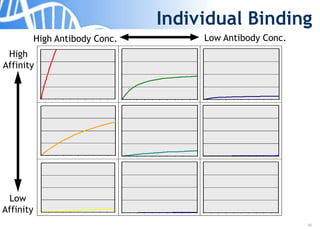
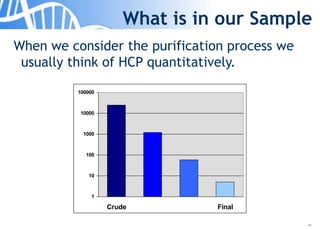
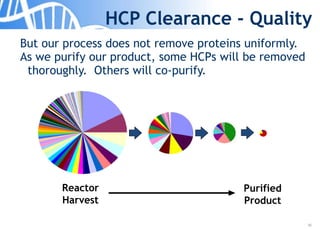
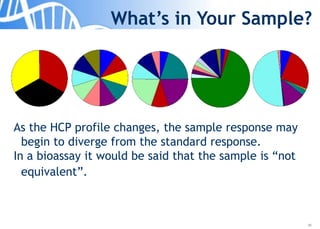
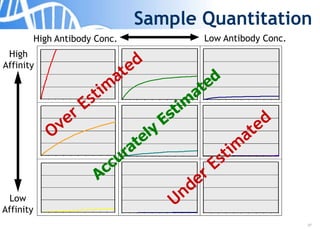
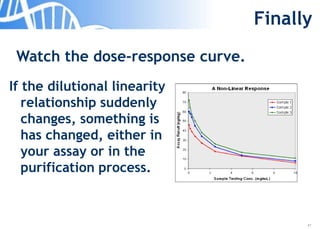
The document discusses the significance of host cell proteins (HCPs) in biopharmaceuticals, highlighting their potential impact on patient safety, product quality, and regulatory compliance. It outlines the challenges of quantifying HCPs, emphasizing the use of immunoassays like ELISA to enhance detection and ensure assay linearity. The text concludes with recommendations for standardized testing practices to overcome existing complications and ensure accuracy in HCP analysis.