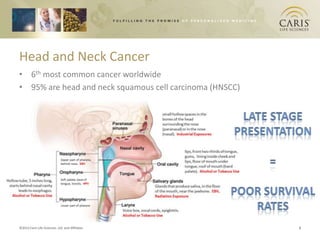
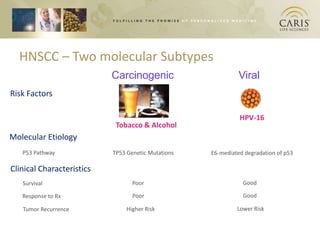
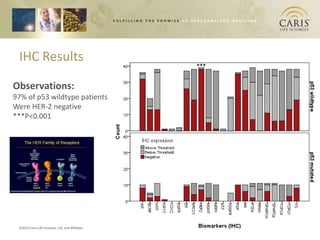
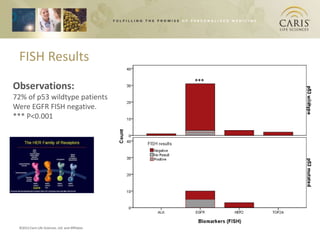
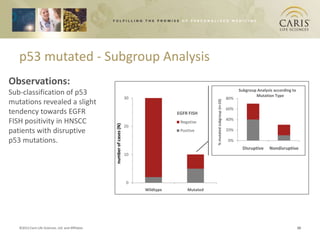
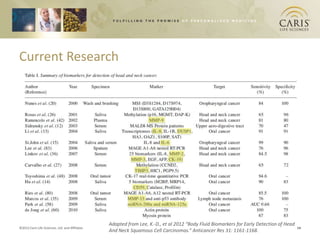
This document summarizes research on differences in biomarker expression between HPV-positive and HPV-negative head and neck cancer subtypes. A study found that 97% of HPV-positive tumors were HER-2 negative compared to HPV-negative tumors, which showed higher EGFR positivity, particularly those with disruptive p53 mutations. This suggests HPV-positive cancers have a better prognosis and may respond to standard therapies, while HPV-negative cancers have a worse prognosis and could benefit from targeted therapies. The document also discusses opportunities for improving early detection through exosome-based liquid biopsies and companies developing diagnostic platforms for head and neck cancer.