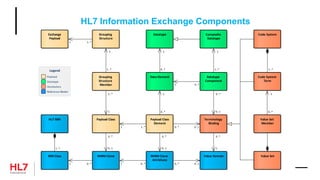
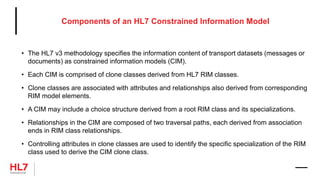
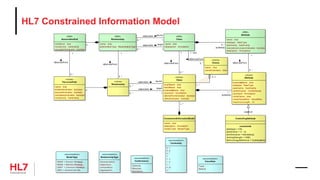
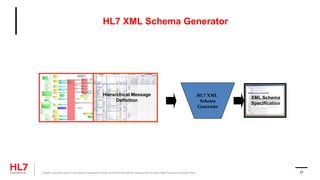
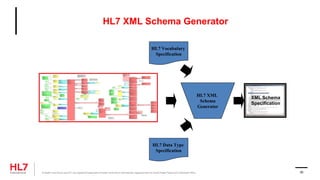
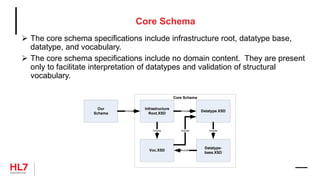
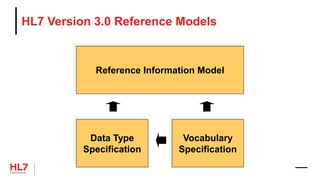
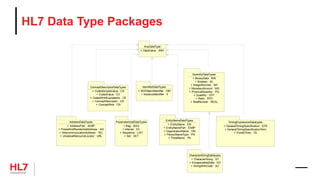
The document provides an outline for a course on the HL7 Clinical Document Architecture (CDA) standard. It includes sections on CDA technical artifacts like the reference information model, vocabulary domains, and data types. It then covers key aspects of the CDA standard specification like the definition of a clinical document and CDA components. The outline also lists learning objectives focused on understanding the CDA standard and creating templates to constrain it for specific use cases.










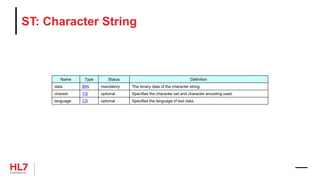
![HL7 V3 Message Design Information Models
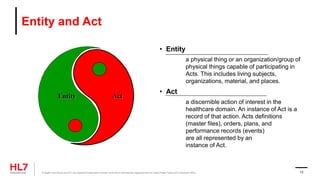
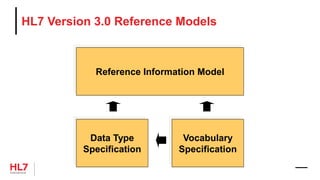
• RIM: Reference Information Model
• D-MIM: Domain Message Information Model
• R-MIM: Refined Message Information Model
• HMD: Hierarchical Message Definition
RIM
Restrict
R-MIM
Serialize
HMD
D-MIM
Derive
PatientIncident
classCode*: <= ENC
moodCode*: <= EVN
id: [1..*] (RegistNum)
code: CV CNE [0..1] <= ExternallyDefinedActCodes (PatientType)
statusCode: LIST<CS> CNE <= ActStatus (IDPHStatus)
activityTime: TS (EDDate)
Injury
classCode*: <= ACT
moodCode*: <= EVN
activityTime: TS (InjuryDate)
0..1 pertinentInjury
typeCode*: <= PERT
pertinentInformation1
TraumaRegistryExport
(IDPH_RM00001)
Data content of HL7
messages used to export
data from the IDPH Trauma
Registry.
PatientPerson
classCode*: <= PSN
determinerCode*: <= INSTANCE
name: PN [0..1] (*Name)
existenceTime: (Age)
administrativeGenderCode: CV CWE <= AdministrativeGender
(GenderID)
birthTime: (DateOfBirth)
addr: AD [0..1] (AddressHome)
raceCode: CV CWE [0..1] <= Race (RaceID)
ethnicGroupCode: CV CWE [0..1] <= Ethnicity (EthnicID)
1..1 patientPatientPerson
1..1 providerTraumaParticipant
Patient
classCode*: <= PAT
id: II [0..1] (MedicaRecordNum)
TraumaParticipant
classCode*: <= ORG
determinerCode*: <= INSTANCE
id: [1..1] (HospitNum)
code: CV CWE [0..1] <= EntityCode
name: ON [0..1] (HospitName)
statusCode: CS CNE [0..1] <= EntityStatus (ActiveFacili)
addr: AD [0..1] (HospitCity)
1..1 patient
typeCode*: <= SBJ
subject
InjuryLocation
classCode*: <= PLC
determinerCode*: <= INSTANCE
code: CV CWE [0..1] <= EntityCode (InjuryPlaceID)
addr: AD [0..1] (AddressScene)
0..1 playingInjuryLocation
Role
classCode*: <= ROL
1..1 participant
typeCode*: <= LOC
location
InjuryRelatedObservation
classCode*: <= OBS
moodCode*: <= EVN
code: <= ExternallyDefinedActCodes
priorityCode: CV CWE [0..1] <= ActPriority
value: [0..1]
0..* pertinentInjuryRelatedObservation
typeCode*: <= PERT
sequenceNumber: INT [0..1] (InjurySequen)
pertinentInformation
Procedure
classCode*: <= PROC
moodCode*: <= EVN
code: CV CWE <= ActCode (ICDCodeID)
activityTime: TS (ProcedDate)
0..* pertinentProcedure
typeCode*: <= PERT
pertinentInformation7
0..1 medicalStaff
typeCode*: <= PRF
performer
MedicalStaff
classCode*: <= PROV
id: II [0..1] (MedicalStaffID)
0..1 procedureLocation
typeCode*: <= LOC
location
ProcedureLocation
classCode*: <= SDLOC
code: <= RoleCode (ProcedLocateID)
PatientIncidentRelatedObservation
classCode*: <= OBS
moodCode*: <= EVN
code: <= ActCode
reasonCode: CV CWE [0..1] <= ActReason
value: ANY [0..1]
0..* pertinentPatientIncidentRelatedObservation
typeCode*: <= PERT
pertinentInformation2
PatientTransfer
classCode*: <= TRNS
moodCode*: <= EVN
activityTime: IVL<TS> (DischaDate to ArriveDate)
reasonCode: CV CWE [0..1] <= TransferActReason (REASONTRANSFID)
1..1 arrivalPatientTransfer
typeCode*: <= ARR
arrivedBy
0..* aRole
typeCode*: <= ORG
origin
0..1 playingTraumaParticipant
aRole
classCode*: <= ROL
TransferRelatedObservation
classCode*: <= OBS
moodCode*: <= EVN
code: CV CWE <= ExternallyDefinedActCodes
value: PQ [0..1]
methodCode: CV CWE [0..1] <= ObservationMethod
1..* pertinentTransferRelatedObservation
typeCode*: <= PERT
pertinentInformation
1..1 transferVehicle
typeCode*: <= VIA
via
1..1 owningVehicleProvider
TransferVehicle
classCode*: <= OWN
id: II [0..1] (VehiclNum)
code: <= RoleCode (VehiclLevelID)
VehicleProvider
classCode*: <= ORG
determinerCode*: <= INSTANCE
id: II [0..1] (VehiclProvide)
code: <= EntityCode (MaxVehiclLevelID)
name: ON [0..1] (VehiclProvidName)
HospitalVisit
classCode*: <= ENC
moodCode*: <= EVN
code: CV CWE <= ActCode (AdmitServicID)
activityTime: TS (DischaDate)
dischargeDispositionCode: CV CWE [0..1]
<= EncounterDischargeDisposition
1..1 pertinentHospitalVisit
typeCode*: <= PERT
pertinentInformation5
HospitalVisitRelatedObservation
classCode*: <= OBS
moodCode*: <= EVN
code: CV CWE <= ExternallyDefinedActCodes
value: [0..1]
0..* pertinentHospitalVisitRelatedObservation
typeCode*: <= PERT
pertinentInformation
1..1 admittingProvider
typeCode*: <= ADM
admitter
0..1 healthCareMedicalStaffPerson
AdmittingProvider
classCode*: <= PROV
id: II [0..1] (ADMITMEDICASTAFFID)
code: CV CWE <= RoleCode (StaffTypeID)
0..* hospitalVisitPhysician
typeCode*: <= RESP
time: TS
responsibleParty
0..1 healthCareMedicalStaffPerson
HospitalVisitPhysician
classCode*: <= PROV
id: II [0..1]
code: CV CWE <= RoleCode (StaffTypeID)
MedicalStaffPerson
classCode*: <= PSN
determinerCode*: <= INSTANCE
name: PN [0..1] (MedicaStaffName)
0..1 licensedEntity
typeCode*: <= DST
destination
0..1 subjectChoice
LicensedEntity
classCode*: <= LIC
id: II [0..1]
Choice
Facility
classCode*: <= ORG
determinerCode*: <= INSTANCE
id:
code*: CS CNE <= EntityCode "FAC"
name:
Hospital
classCode*: <= ORG
determinerCode*: <= INSTANCE
id:
code*: CS CNE <= EntityCode "HOSP"
name:
EmergencyDepartmentEncounter
classCode*: <= ENC
moodCode*: <= EVN
activityTime: IVL<TS>
dischargeDispositionCode: CV CWE <= EncounterDischargeDisposition
0..1 pertinentEmergencyDepartmentEncounter
typeCode*: <= PERT
pertinentInformation3
EmergencyDepartmentRelatedObservation
classCode*: <= OBS
moodCode*: <= EVN
code: CV CWE <= ExternallyDefinedActCodes
text:
activityTime: TS
reasonCode: <= ActReason
value: [0..1]
methodCode: CV CWE [0..1] <= ObservationMethod
targetSiteCode: CV CWE [0..1] <= HumanActSite
0..* pertinentEmergencyDepartmentRelatedObservation
typeCode*: <= PERT
pertinentInformation
0..* emergencyDepartmentPhysician
typeCode*: <= PRF
performer
0..1 healthCareMedicalStaffPerson EmergencyDepartmentPhysician
classCode*: <= PROV
id: II [0..1]
code: CE CWE [0..1] <= RoleCode (StaffTypeID)
PreHospitalEncounter
classCode*: <= ENC
moodCode*: <= EVN
id: II [0..1] (crashNum)
activityTime: IVL<TS>
0..1 priorPreHospitalEncounter
typeCode*: <= PREV
predecessor
PreHosptialRelatedObservation
classCode*: <= OBS
moodCode*: <= EVN
code: <= ExternallyDefinedActCodes
value: ANY [0..1]
0..* pertinentPreHosptialRelatedObservation
typeCode*: <= PERT
pertinentInformation
1..1 preHospitalVehicle
typeCode*: <= ParticipationType
participant
1..1 owningVehicleProvider
PreHospitalVehicle
classCode*: <= OWN
id: II [0..1] (VehiclNum)
code: <= RoleCode (VehiclLevelID)
0..* emergencyDepartmentPhysicianAct
typeCode*: <= COMP
component
EmergencyDepartmentPhysicianAct
classCode*: <= ACT
moodCode*: <= EVN
code: CS CNE [0..1] <= ExternallyDefinedActCodes
activityTime*: TS [0..1]
component
0..* patientIncidentRelatedObservation
typeCode*: <= COMP
VehicleProvider
MedicalStaffPerson
TraumaParticipant
A_AbnormalityAssessment
(COCT_RM420000UV)
Description: assessment of clinical findings, including lab test results,
for indications of the presence and severity of abnormal conditions
AbnormalityAssessment
classCode*: = "OBS"
moodCode*: = "EVN"
code*: CD CWE [1..1] <= V:ObservationType ("ADVERSE_REACTION")
statusCode*: CS CNE [1..1] <= V:ActStatusAbortedCancelledCompleted
activityTime*: TS.DATETIME [1..1]
value: CD CWE [0..1] <= V:AbnormalityAssessmentValue
methodCode: SET<CE> CWE [0..*] <= V:AbnormalityAssessmentMethod
1..* assessmentOutcome *
typeCode*: = "OUTC"
contextConductionInd*: BL [1..1] ="true"
outcome
AssessmentException
classCode*: = "OBS"
moodCode*: = "EVN"
code*: CD CWE [1..1] <= V:ObservationType ("ASSERTION")
value*: SC CWE [1..1] <= V:AssessmentExceptionValue
AbnormalityGrade
classCode*: = "OBS"
moodCode*: = "EVN"
code*: CD CWE [1..1] <= V:ObservationType ("SEV")
uncertaintyCode: CE CNE [0..1] <= V:ActUncertainty
value*: CD CWE [1..1] <= V:AbnormalityGradeValue
AssessmentOutcome
0..* assessmentOutcomeAnnotation
typeCode*: = "APND"
contextConductionInd*: BL [1..1] ="true"
appendageOf
AssessmentOutcomeAnnotation
classCode*: = "OBS"
moodCode*: = "EVN"
code*: CD CWE [1..1] <= V:ObservationType ("ASSERTION")
value*: SC CWE [1..1] <= V:AssessmentOutcomeAnnotationValue
® Health Level Seven and HL7 are registered trademarks of Health Level Seven International, registered with the United States Patent and Trademark Office. 16](https://image.slidesharecdn.com/hl7advancecdamay2019webinar-190521131121/85/Hl7-advance-cda-may-2019-webinar-16-320.jpg)
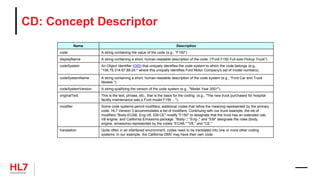
![• Constrained Information Model (CIM)
HL7 V3 Contrained Information Models
• RIM: Reference Information Model
• D-MIM: Domain Message Information Model
• R-MIM: Refined Message Information Model
• HMD: Hierarchical Message Definition
RIM
Restrict
R-MIM
Serialize
HMD
D-MIM
Derive
PatientIncident
classCode*: <= ENC
moodCode*: <= EVN
id: [1..*] (RegistNum)
code: CV CNE [0..1] <= ExternallyDefinedActCodes (PatientType)
statusCode: LIST<CS> CNE <= ActStatus (IDPHStatus)
activityTime: TS (EDDate)
Injury
classCode*: <= ACT
moodCode*: <= EVN
activityTime: TS (InjuryDate)
0..1 pertinentInjury
typeCode*: <= PERT
pertinentInformation1
TraumaRegistryExport
(IDPH_RM00001)
Data content of HL7
messages used to export
data from the IDPH Trauma
Registry.
PatientPerson
classCode*: <= PSN
determinerCode*: <= INSTANCE
name: PN [0..1] (*Name)
existenceTime: (Age)
administrativeGenderCode: CV CWE <= AdministrativeGender
(GenderID)
birthTime: (DateOfBirth)
addr: AD [0..1] (AddressHome)
raceCode: CV CWE [0..1] <= Race (RaceID)
ethnicGroupCode: CV CWE [0..1] <= Ethnicity (EthnicID)
1..1 patientPatientPerson
1..1 providerTraumaParticipant
Patient
classCode*: <= PAT
id: II [0..1] (MedicaRecordNum)
TraumaParticipant
classCode*: <= ORG
determinerCode*: <= INSTANCE
id: [1..1] (HospitNum)
code: CV CWE [0..1] <= EntityCode
name: ON [0..1] (HospitName)
statusCode: CS CNE [0..1] <= EntityStatus (ActiveFacili)
addr: AD [0..1] (HospitCity)
1..1 patient
typeCode*: <= SBJ
subject
InjuryLocation
classCode*: <= PLC
determinerCode*: <= INSTANCE
code: CV CWE [0..1] <= EntityCode (InjuryPlaceID)
addr: AD [0..1] (AddressScene)
0..1 playingInjuryLocation
Role
classCode*: <= ROL
1..1 participant
typeCode*: <= LOC
location
InjuryRelatedObservation
classCode*: <= OBS
moodCode*: <= EVN
code: <= ExternallyDefinedActCodes
priorityCode: CV CWE [0..1] <= ActPriority
value: [0..1]
0..* pertinentInjuryRelatedObservation
typeCode*: <= PERT
sequenceNumber: INT [0..1] (InjurySequen)
pertinentInformation
Procedure
classCode*: <= PROC
moodCode*: <= EVN
code: CV CWE <= ActCode (ICDCodeID)
activityTime: TS (ProcedDate)
0..* pertinentProcedure
typeCode*: <= PERT
pertinentInformation7
0..1 medicalStaff
typeCode*: <= PRF
performer
MedicalStaff
classCode*: <= PROV
id: II [0..1] (MedicalStaffID)
0..1 procedureLocation
typeCode*: <= LOC
location
ProcedureLocation
classCode*: <= SDLOC
code: <= RoleCode (ProcedLocateID)
PatientIncidentRelatedObservation
classCode*: <= OBS
moodCode*: <= EVN
code: <= ActCode
reasonCode: CV CWE [0..1] <= ActReason
value: ANY [0..1]
0..* pertinentPatientIncidentRelatedObservation
typeCode*: <= PERT
pertinentInformation2
PatientTransfer
classCode*: <= TRNS
moodCode*: <= EVN
activityTime: IVL<TS> (DischaDate to ArriveDate)
reasonCode: CV CWE [0..1] <= TransferActReason (REASONTRANSFID)
1..1 arrivalPatientTransfer
typeCode*: <= ARR
arrivedBy
0..* aRole
typeCode*: <= ORG
origin
0..1 playingTraumaParticipant
aRole
classCode*: <= ROL
TransferRelatedObservation
classCode*: <= OBS
moodCode*: <= EVN
code: CV CWE <= ExternallyDefinedActCodes
value: PQ [0..1]
methodCode: CV CWE [0..1] <= ObservationMethod
1..* pertinentTransferRelatedObservation
typeCode*: <= PERT
pertinentInformation
1..1 transferVehicle
typeCode*: <= VIA
via
1..1 owningVehicleProvider
TransferVehicle
classCode*: <= OWN
id: II [0..1] (VehiclNum)
code: <= RoleCode (VehiclLevelID)
VehicleProvider
classCode*: <= ORG
determinerCode*: <= INSTANCE
id: II [0..1] (VehiclProvide)
code: <= EntityCode (MaxVehiclLevelID)
name: ON [0..1] (VehiclProvidName)
HospitalVisit
classCode*: <= ENC
moodCode*: <= EVN
code: CV CWE <= ActCode (AdmitServicID)
activityTime: TS (DischaDate)
dischargeDispositionCode: CV CWE [0..1]
<= EncounterDischargeDisposition
1..1 pertinentHospitalVisit
typeCode*: <= PERT
pertinentInformation5
HospitalVisitRelatedObservation
classCode*: <= OBS
moodCode*: <= EVN
code: CV CWE <= ExternallyDefinedActCodes
value: [0..1]
0..* pertinentHospitalVisitRelatedObservation
typeCode*: <= PERT
pertinentInformation
1..1 admittingProvider
typeCode*: <= ADM
admitter
0..1 healthCareMedicalStaffPerson
AdmittingProvider
classCode*: <= PROV
id: II [0..1] (ADMITMEDICASTAFFID)
code: CV CWE <= RoleCode (StaffTypeID)
0..* hospitalVisitPhysician
typeCode*: <= RESP
time: TS
responsibleParty
0..1 healthCareMedicalStaffPerson
HospitalVisitPhysician
classCode*: <= PROV
id: II [0..1]
code: CV CWE <= RoleCode (StaffTypeID)
MedicalStaffPerson
classCode*: <= PSN
determinerCode*: <= INSTANCE
name: PN [0..1] (MedicaStaffName)
0..1 licensedEntity
typeCode*: <= DST
destination
0..1 subjectChoice
LicensedEntity
classCode*: <= LIC
id: II [0..1]
Choice
Facility
classCode*: <= ORG
determinerCode*: <= INSTANCE
id:
code*: CS CNE <= EntityCode "FAC"
name:
Hospital
classCode*: <= ORG
determinerCode*: <= INSTANCE
id:
code*: CS CNE <= EntityCode "HOSP"
name:
EmergencyDepartmentEncounter
classCode*: <= ENC
moodCode*: <= EVN
activityTime: IVL<TS>
dischargeDispositionCode: CV CWE <= EncounterDischargeDisposition
0..1 pertinentEmergencyDepartmentEncounter
typeCode*: <= PERT
pertinentInformation3
EmergencyDepartmentRelatedObservation
classCode*: <= OBS
moodCode*: <= EVN
code: CV CWE <= ExternallyDefinedActCodes
text:
activityTime: TS
reasonCode: <= ActReason
value: [0..1]
methodCode: CV CWE [0..1] <= ObservationMethod
targetSiteCode: CV CWE [0..1] <= HumanActSite
0..* pertinentEmergencyDepartmentRelatedObservation
typeCode*: <= PERT
pertinentInformation
0..* emergencyDepartmentPhysician
typeCode*: <= PRF
performer
0..1 healthCareMedicalStaffPerson EmergencyDepartmentPhysician
classCode*: <= PROV
id: II [0..1]
code: CE CWE [0..1] <= RoleCode (StaffTypeID)
PreHospitalEncounter
classCode*: <= ENC
moodCode*: <= EVN
id: II [0..1] (crashNum)
activityTime: IVL<TS>
0..1 priorPreHospitalEncounter
typeCode*: <= PREV
predecessor
PreHosptialRelatedObservation
classCode*: <= OBS
moodCode*: <= EVN
code: <= ExternallyDefinedActCodes
value: ANY [0..1]
0..* pertinentPreHosptialRelatedObservation
typeCode*: <= PERT
pertinentInformation
1..1 preHospitalVehicle
typeCode*: <= ParticipationType
participant
1..1 owningVehicleProvider
PreHospitalVehicle
classCode*: <= OWN
id: II [0..1] (VehiclNum)
code: <= RoleCode (VehiclLevelID)
0..* emergencyDepartmentPhysicianAct
typeCode*: <= COMP
component
EmergencyDepartmentPhysicianAct
classCode*: <= ACT
moodCode*: <= EVN
code: CS CNE [0..1] <= ExternallyDefinedActCodes
activityTime*: TS [0..1]
component
0..* patientIncidentRelatedObservation
typeCode*: <= COMP
VehicleProvider
MedicalStaffPerson
TraumaParticipant
A_AbnormalityAssessment
(COCT_RM420000UV)
Description: assessment of clinical findings, including lab test results,
for indications of the presence and severity of abnormal conditions
AbnormalityAssessment
classCode*: = "OBS"
moodCode*: = "EVN"
code*: CD CWE [1..1] <= V:ObservationType ("ADVERSE_REACTION")
statusCode*: CS CNE [1..1] <= V:ActStatusAbortedCancelledCompleted
activityTime*: TS.DATETIME [1..1]
value: CD CWE [0..1] <= V:AbnormalityAssessmentValue
methodCode: SET<CE> CWE [0..*] <= V:AbnormalityAssessmentMethod
1..* assessmentOutcome *
typeCode*: = "OUTC"
contextConductionInd*: BL [1..1] ="true"
outcome
AssessmentException
classCode*: = "OBS"
moodCode*: = "EVN"
code*: CD CWE [1..1] <= V:ObservationType ("ASSERTION")
value*: SC CWE [1..1] <= V:AssessmentExceptionValue
AbnormalityGrade
classCode*: = "OBS"
moodCode*: = "EVN"
code*: CD CWE [1..1] <= V:ObservationType ("SEV")
uncertaintyCode: CE CNE [0..1] <= V:ActUncertainty
value*: CD CWE [1..1] <= V:AbnormalityGradeValue
AssessmentOutcome
0..* assessmentOutcomeAnnotation
typeCode*: = "APND"
contextConductionInd*: BL [1..1] ="true"
appendageOf
AssessmentOutcomeAnnotation
classCode*: = "OBS"
moodCode*: = "EVN"
code*: CD CWE [1..1] <= V:ObservationType ("ASSERTION")
value*: SC CWE [1..1] <= V:AssessmentOutcomeAnnotationValue
® Health Level Seven and HL7 are registered trademarks of Health Level Seven International, registered with the United States Patent and Trademark Office. 19](https://image.slidesharecdn.com/hl7advancecdamay2019webinar-190521131121/85/Hl7-advance-cda-may-2019-webinar-19-320.jpg)










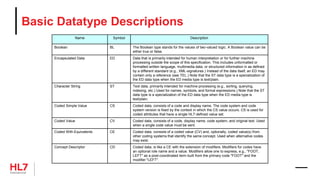
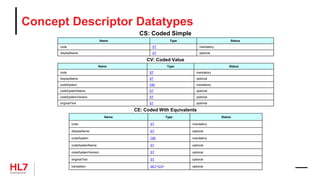
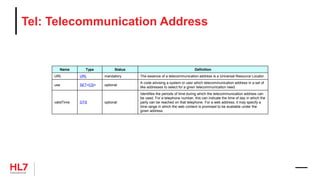

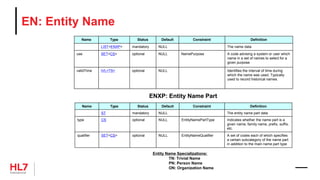

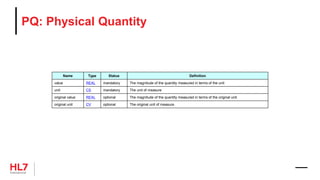
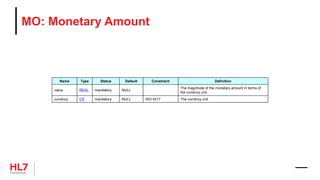
















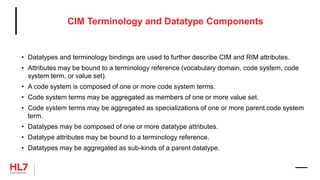
![HL7 CIM Terminology and Datatype
«clone»
Attribute
businessName: char
datatype: DataType
cardinality: Cardinality
conformance: Conformance
comment: Annotation
initialValue: char
initialValueRole: ValueRole
maximumLength: int
«RIM»
Attribute
name: char
datatype: DataType
cardinality: Cardinality
mandatoryInclusionIndicator: boolean
description: Annotation
ControllingAttribute
constraints
{datatype = CS}
{cardinality = (1..1)}
{conformance = Mandatory}
{codingStrength = CNE}
{terminologyReference = CodeSystem}
«RIM»
TerminologyBinding
codingStrength: CodingStrength
«clone»
TerminologyBinding
codingStrength: CodingStrength
TerminologyReference
{abstract}
name: char
description: Annotation
VocabularyDomain
ValueSet
conceptIdentifier: char
CodeSystem
objectIdentifier: char
releaseIdentifier: char
isExternalIndicator: boolean
CodeSystemTerm
code: char [0..1]
designation: char
description: Annotation
internalIdentifier: char
DataType
longName: char
shortName: char
description: Annotation
DatatypeAttribute
name: char
datatype: DataType
description: Annotation
ParentDatatype
«datatype»
TerminologyBinding
codingStrength: CodingStrength
Annotation
{abstract}
«enumeration»
CodingStrength
CNE
CWE
ParentCodeSystemTerm
AnnotationSection
sectionRole: AnnotationSectionRole
sectionText: char
«enumeration»
AnnotationSectionRole
Description
Rationale
Design Comment
Issue
Implementation Note
History
Mapping
0..*
binds
1
0..1
binds
1
0..1
binds
1
0..*
isDerivedFrom
1
«restrict»
0..*
isDerivedFrom
1
0..*
binds
1
1..*
subkind
1..*
0..*
0..*
binds
1
0..1
binds
1
0..*
0..*
isBoundTo
1
member
1..*0..*
1..*
subkind
1..*
0..1](https://image.slidesharecdn.com/hl7advancecdamay2019webinar-190521131121/85/Hl7-advance-cda-may-2019-webinar-75-320.jpg)

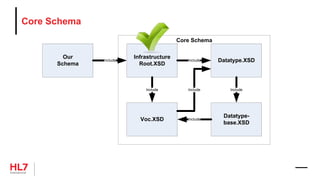

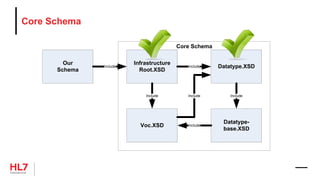

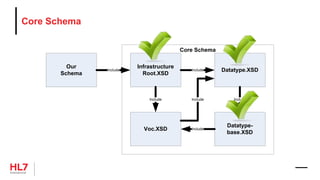


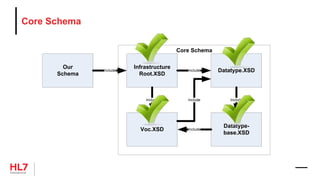













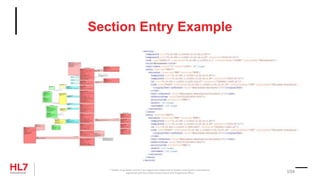

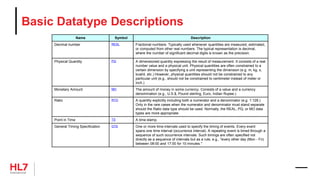
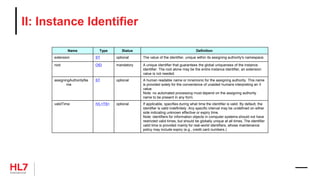
![Template Conformance Statement
SHALL contain exactly one [1..1] @classCode="OBS" Observation
(CodeSystem: HL7ActClass 2.16.840.1.113883.5.6 STATIC)
Element: @classCode
Usage: SHALL contain
Cardinality: exactly one [1..1]
Terminology Binding: ="OBS" Observation
(CodeSystem: HL7ActClass 2.16.840.1.113883.5.6 STATIC)
® Health Level Seven and HL7 are registered trademarks of Health Level Seven International, registered with the United States Patent and Trademark Office. 106](https://image.slidesharecdn.com/hl7advancecdamay2019webinar-190521131121/85/Hl7-advance-cda-may-2019-webinar-106-320.jpg)










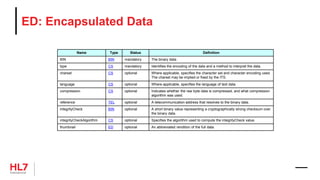
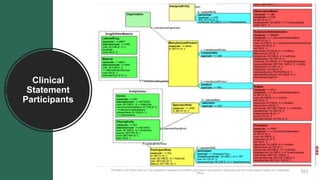
![Medication Activity
Entry Template
(required elements only)
• SHALL contain exactly one [1..1] @classCode="SBADM".
• SHALL contain exactly one [1..1] @moodCode=MoodCodeEvnInt.
• SHALL contain exactly one [1..1] templateId.
• @root=“2.16.840.1.113883.10.20.22.4.16”
• @extension=“2014-06-09”
• SHALL contain at least one [1..*] id.
• SHALL contain exactly one [1..1] statusCode=Medication Status.
• SHALL contain exactly one [1..1] effectiveTime.
• SHOULD contain zero or one [0..1] @value.
• SHOULD contain zero or one [0..1] low.
• MAY contain zero or one [0..1] high.
• SHALL contain either a low or a @value but not both.
• SHALL contain exactly one [1..1] doseQuantity.
• SHALL contain exactly one [1..1] consumable
(Medication Information).
«Substance Administration»
Medication Activity
- @classCode: CD ="SBADM"
- @moodCode: CD =MoodCodeEvnInt
- templateID.@root: ST=“2.16.840.1.113...
- templateID.@extension: ST=“2014-06-09”
- id: II
- statusCode: CD =MedicationStatus
- effectiveTime.@value: TS [0..1]
- effectiveTime.low: TS [0..1]
- effectiveTime.high: TS [0..1]
- doseQuantity: PQ
«Participation»
Consumable
- typeCode: CD ="CSM"
«Role»
Manufactured Product
- @classCode: CD ="MANU"
- id: II [0..*](SET)
«Manufactured Material»
Material
- @classCode: CD ="MMAT"
- @determinerCode: CD ="KIND"
- code: CD =MedicationClini...
- name: EN [0..1]
- lotNumberText: ST[0..1]
«Organization»
Manufacturer Organization
- @classCode: CD ="ORG"
- @determinerCode: CD ="Instance"
- id: II [1..*](SET)
- name: EN.ON
1..11..1
player
+manufacturedDrugOrOtherMaterial 1..1
scoper
+manufacturer 0..1](https://image.slidesharecdn.com/hl7advancecdamay2019webinar-190521131121/85/Hl7-advance-cda-may-2019-webinar-121-320.jpg)