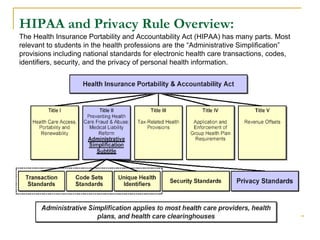
The document provides an overview of the Health Insurance Portability and Accountability Act (HIPAA) for health care professionals. Some key points:
- HIPAA aims to protect patients' protected health information (PHI) and set standards for handling electronic health data.
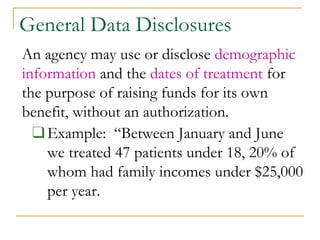
- PHI includes any individually identifiable health information like names, birthdates, diagnoses. Healthcare workers may only access and share PHI as needed for treatment, payment or operations.
- Permitted uses of PHI include treatment, payment, health operations. Disclosures require patient authorization except as required by law like public health reporting. Incidental disclosures must be limited in nature.
- Violations can result in fines or imprisonment.