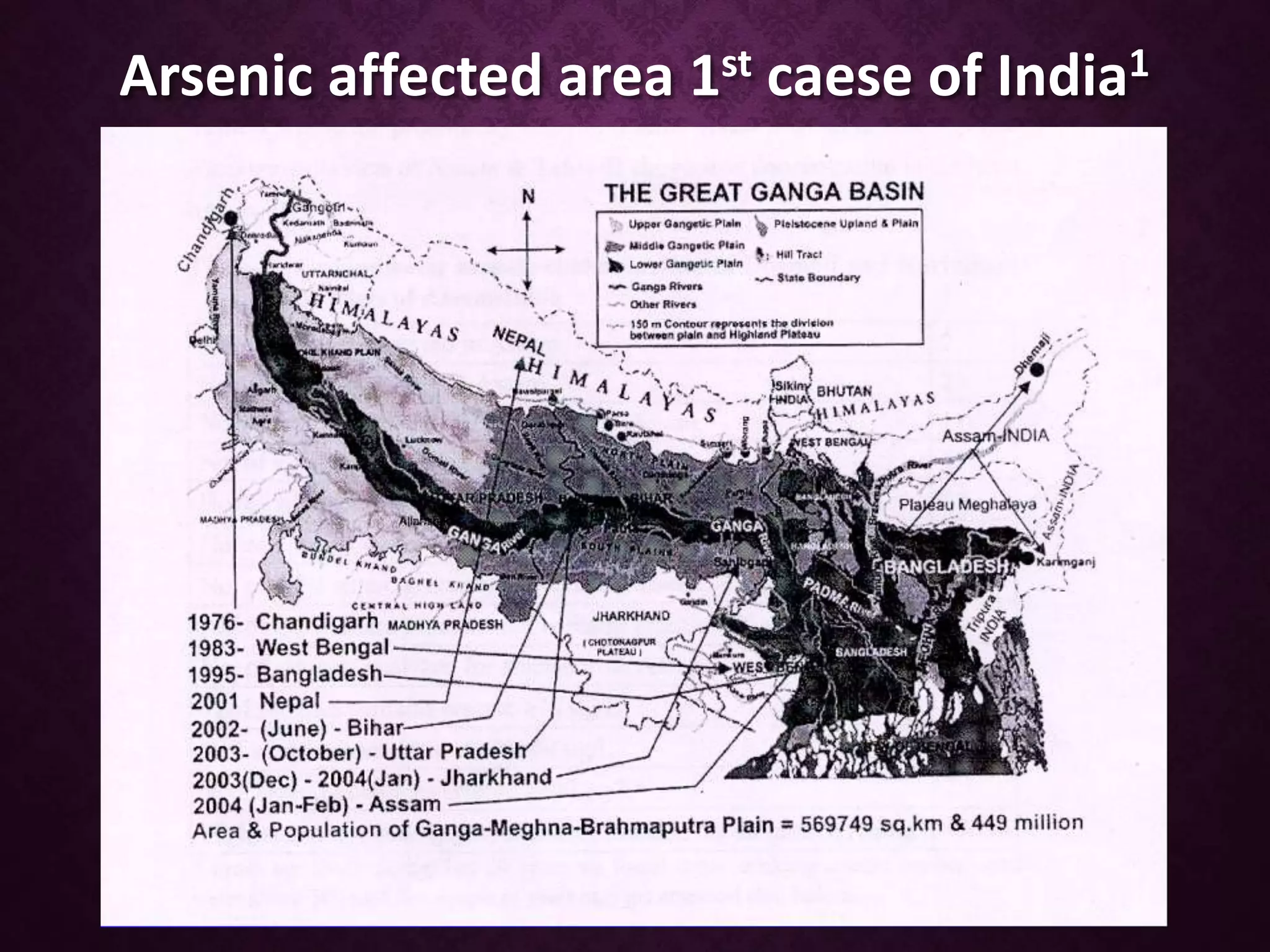
The document discusses the severe issue of groundwater arsenic contamination in India, its chemistry, extent, and various remedial measures. Key findings reveal affected regions, mainly in West Bengal and Bihar, where arsenic levels exceed permissible limits, and outlines numerous research studies related to health impacts and remediation techniques. It highlights effective methods for arsenic removal from water, including oxidation, coagulants, adsorption, and advanced technologies like reverse osmosis.


![Chemistry of Arsenic (1/4)
• Atomic number: 33
• Atomic wt.: 74.9
• Isotope: 3 (73,74,75)
• Electron configuration: [Ar] 3d10 4s2 4p3
• Non-magnetic, semi-metal
• Colour: Gray and Black
• Hardness: 3.5
• Oxidation state: 5, 3, -3](https://image.slidesharecdn.com/groundwaterarsenicindia-180627111359/75/Ground-water-Arsenic-Contamination-in-India-3-2048.jpg)
























![Remedial measures: Coagulation with lime
Chemical used: Quick lime (CaO) or hydrated lime [Ca(OH)2]
Process: similar like metal slat process of coagulation
Calcium hydroxide acts as a sorptive flocculant for arsenic
Excess lime and precipitate removed: sedimentation & filtration
Works in pH 10.6-11.4
Removes 40-70% arsenic
Better to use pre-treatment process for alum & iron coagulation](https://image.slidesharecdn.com/groundwaterarsenicindia-180627111359/75/Ground-water-Arsenic-Contamination-in-India-29-2048.jpg)
![Remedial measures: Solar oxidation & precipitation of
Iron(III) with adsorbed Arsenic(V) [SORAS]
Photochemical oxidation: Irradiation of water in PET or
other UV-A transparent bottle- coverts As(III) to As(V)
Precipitation or filtration of adsorbed As(V) on Fe(III)-
oxides [Fe naturally present or added]
A household method where ground water naturally
contains Fe(II) and Fe(III)](https://image.slidesharecdn.com/groundwaterarsenicindia-180627111359/75/Ground-water-Arsenic-Contamination-in-India-30-2048.jpg)

![Remedial measures: Adsorption (Sorptive
filtration) using activated alumina [Al2O3]
Good sorptive surface: 200-300 m2/gm
water passed through packed column of
alumina- impurities and As retaians
Caustic soda (NaOH) used to regenerate
saturated packed column of alumina
Example: BUET Activated Alumina, Alcan
Enhanced Activate Alumina, Apyron Arsenic
Treatment Unit](https://image.slidesharecdn.com/groundwaterarsenicindia-180627111359/75/Ground-water-Arsenic-Contamination-in-India-32-2048.jpg)













