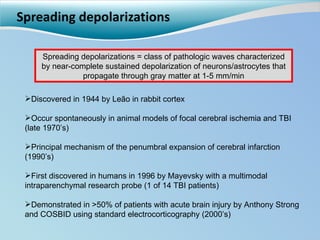
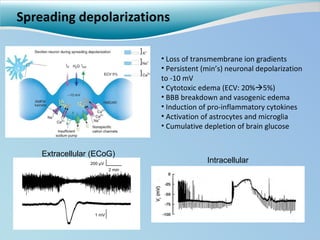
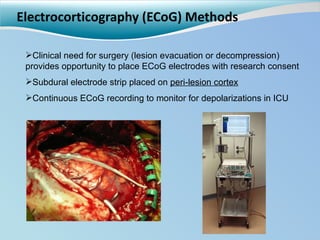
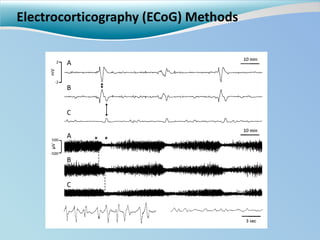
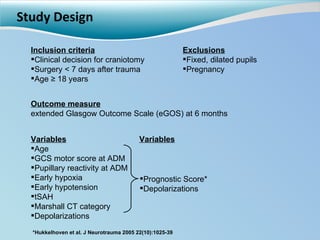
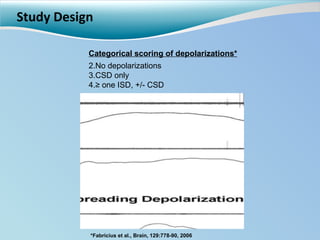
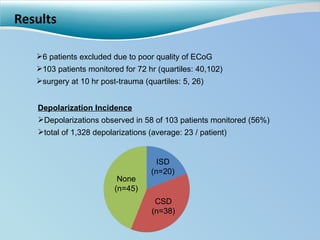
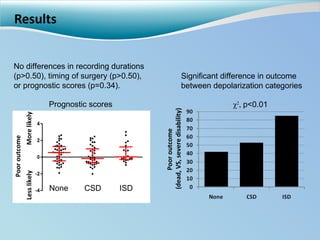
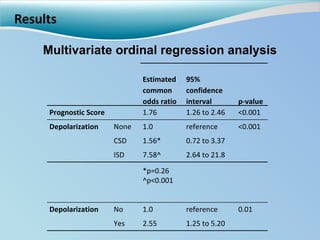
Cortical spreading depolarizations are waves of neuronal and astrocyte depolarization that propagate through brain tissue and are associated with worse clinical outcomes after traumatic brain injury. The study found that over 50% of patients with brain injury experienced spreading depolarizations when monitored with electrocorticography. In a multivariate analysis controlling for established prognostic factors, spreading depolarizations remained independently associated with unfavorable six-month outcomes. The results suggest spreading depolarizations are a pathomechanism with adverse effects on the injured brain that could potentially be targeted by new therapies.
![Cortical spreading depolarizations are a novel mechanism independently associated with unfavorable clinical outcome in TBI Jed Hartings, PhD Department of Neurosurgery University of Cincinnati [email_address] 513-558-3567 COSBID](https://image.slidesharecdn.com/hartingsoutcome-110811153518-phpapp01/75/Hartings-Jed-Outcome-1-2048.jpg)