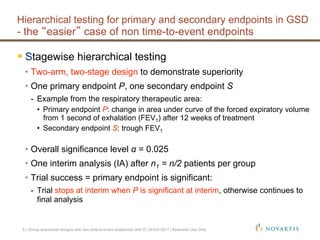
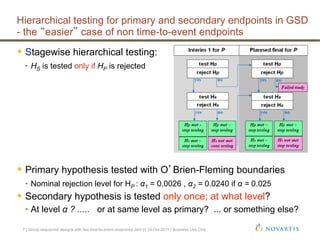
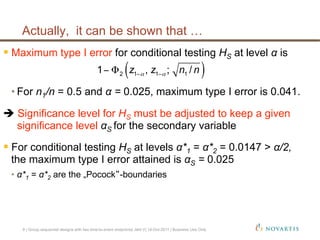
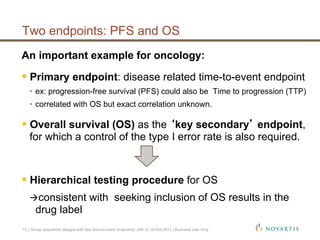
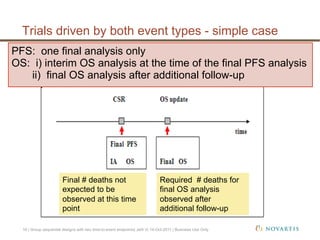
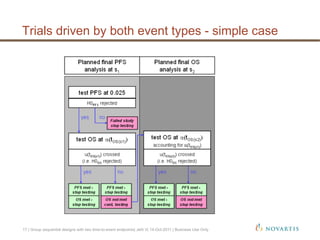
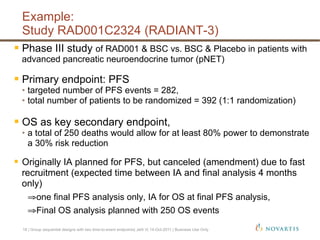
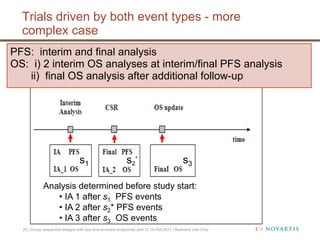
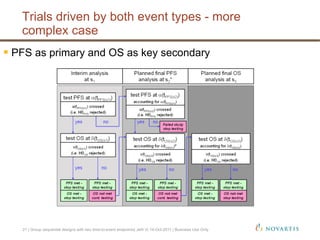
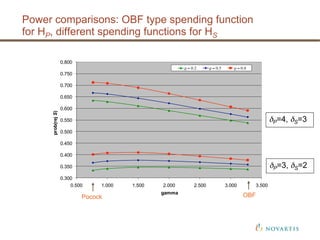
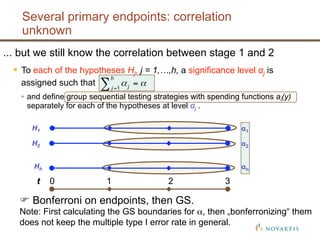
This document discusses approaches for handling multiple time-to-event endpoints in group sequential clinical trial designs. It provides examples of hierarchical testing procedures where the secondary endpoint is only evaluated if the primary endpoint is significant. It also discusses approaches where trials are driven by both primary and secondary event types, with interim analyses planned for each endpoint. Maintaining control of the overall type I error rate across multiple analyses and endpoints is an important consideration.