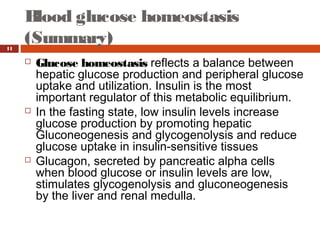
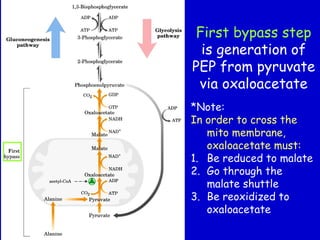
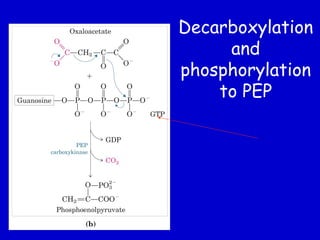
Homeostasis refers to the maintenance of stable internal conditions in the body. Glucose homeostasis specifically reflects a balance between hepatic glucose production and peripheral glucose uptake, regulated by insulin and glucagon. In the fasting state, low insulin and high glucagon promote gluconeogenesis and glycogenolysis in the liver to produce glucose for tissues like the brain that require it. Postprandially, high insulin and low glucagon stimulate glucose uptake in tissues and inhibit hepatic glucose production. Gluconeogenesis allows the conversion of substrates like lactate, glycerol, and amino acids into glucose, especially in the liver. It bypasses three irreversible glycolytic steps through key enzymes. The Cori and Alanine cycles shuttle lact