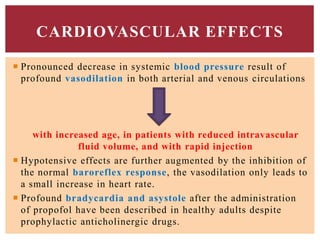
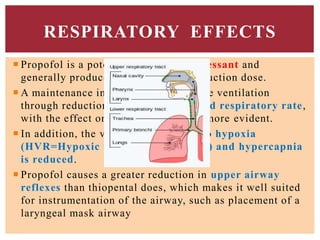
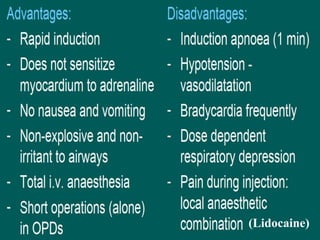
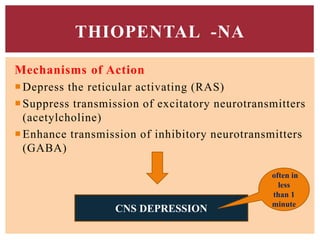
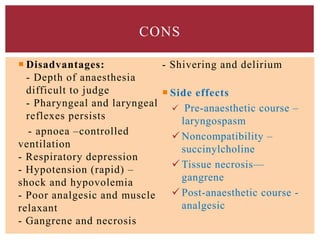
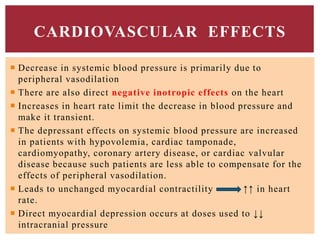
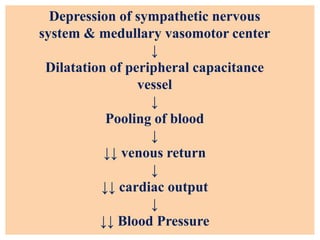
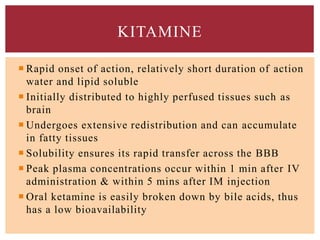
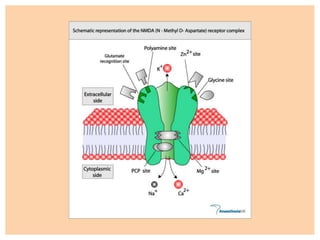
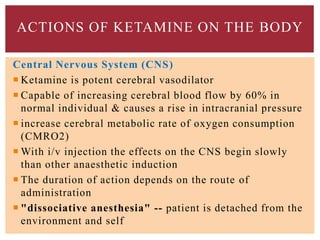
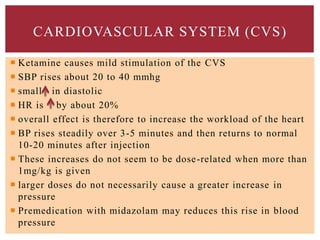
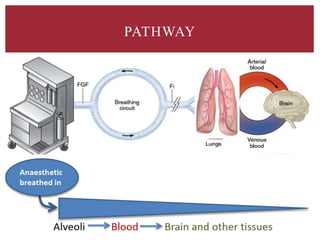
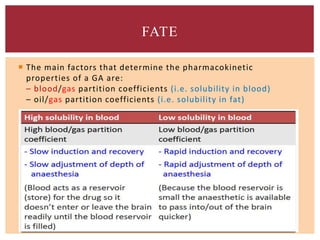
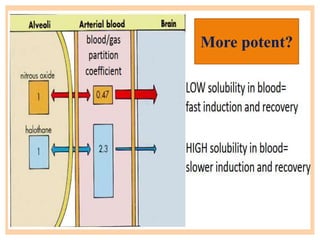
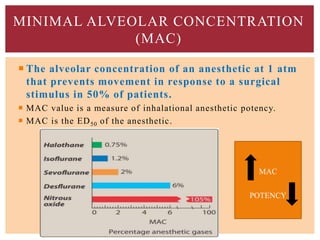
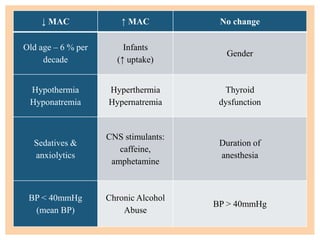
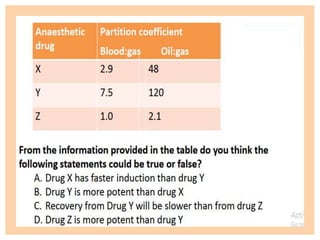
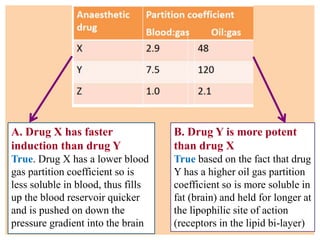
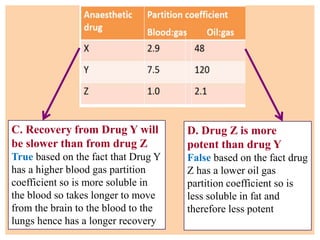
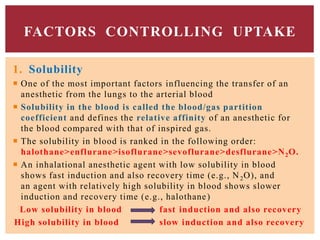
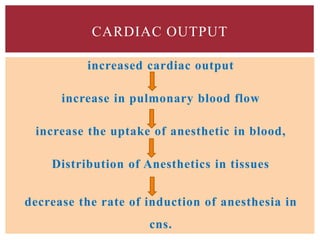
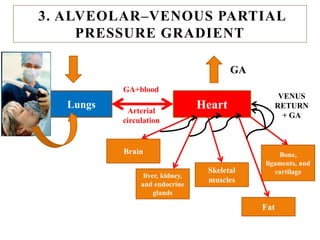
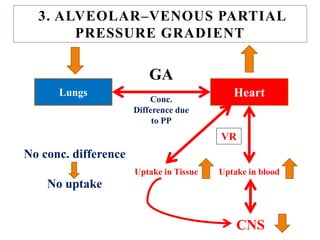
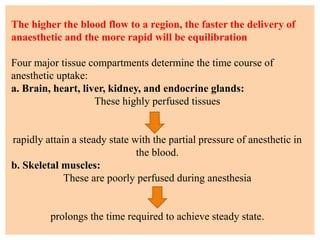
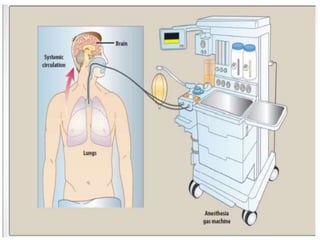
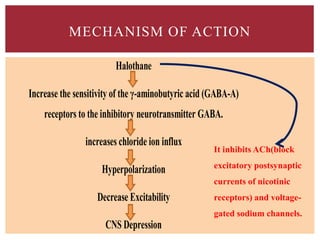
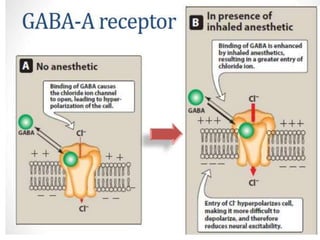
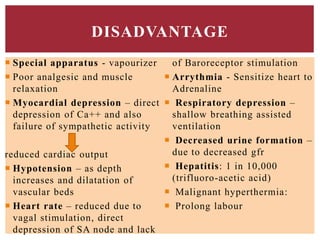
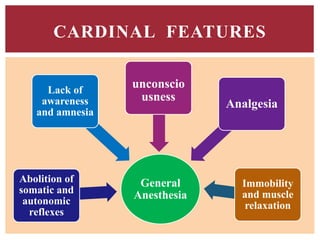
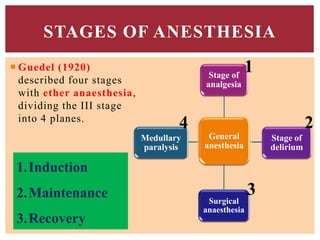
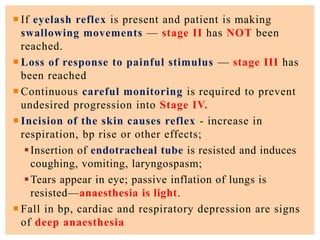
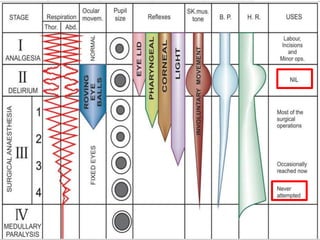
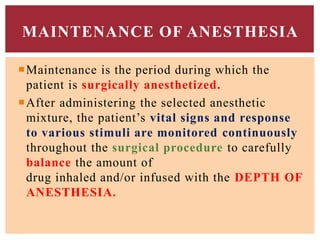
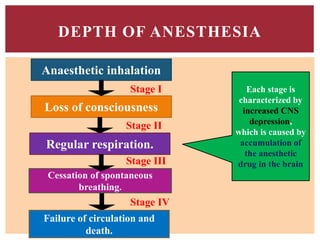
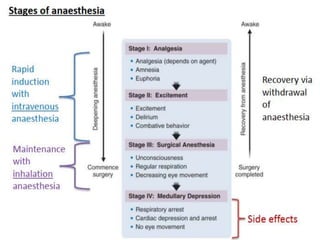
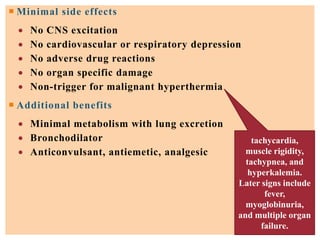
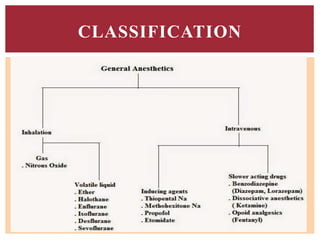
The document discusses general anesthetics, emphasizing their role in inducing a controlled loss of sensation for medical procedures. It outlines the characteristics, advantages, disadvantages, and stages of general anesthesia, as well as the importance of pre-anesthetic assessments and the management of potential complications. It also describes the use of intravenous anesthetics, specifically propofol, noting its effects, mechanisms, and associated risks such as propofol infusion syndrome.





















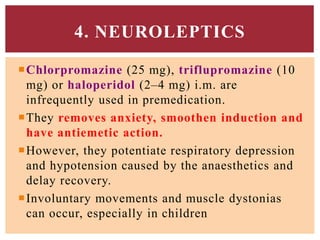




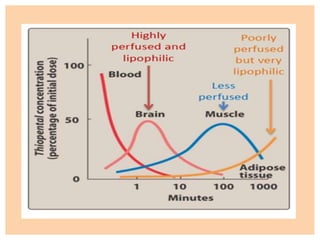
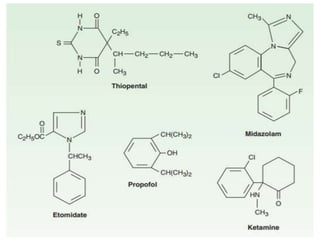
![Propofol [PROPE-o-fol] is an IV
sedative/hypnotic used in the induction or
maintenance of anesthesia.
Propofol is widely used and has replaced
thiopental as the first choice for anesthesia
induction and sedation, because it produces a
euphoric feeling in the patient and does not
cause postanesthetic nausea and vomiting.
PROPOFOL](https://image.slidesharecdn.com/generalanesthetics-190726052619/85/General-anesthetics-44-320.jpg)