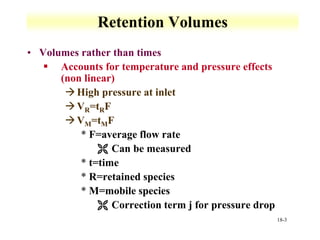
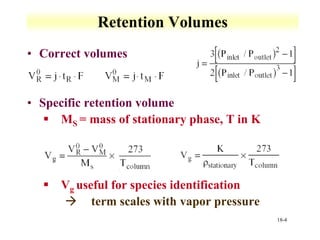
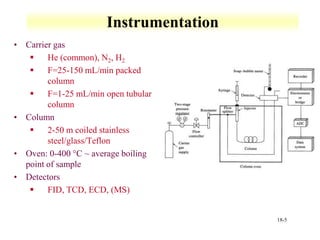
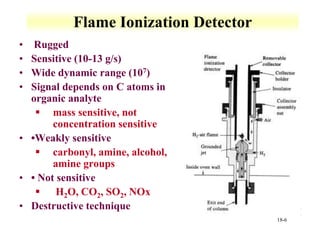
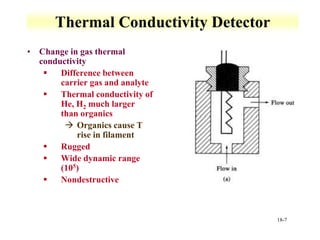
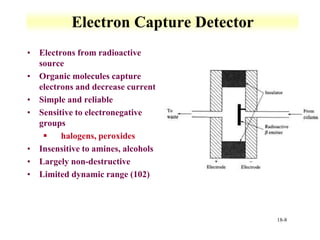
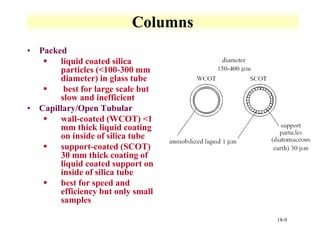
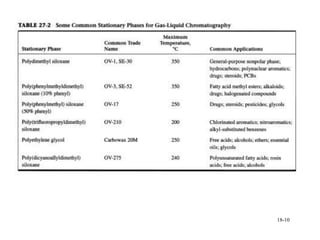
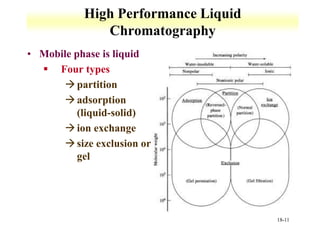
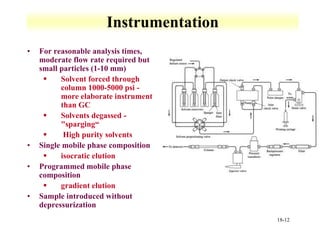
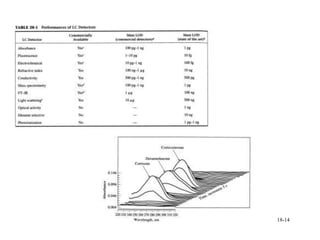
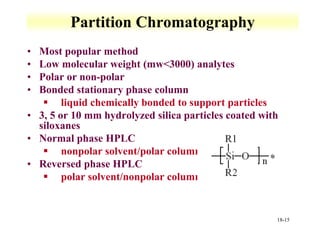
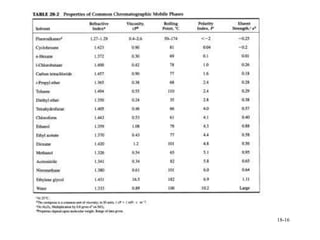
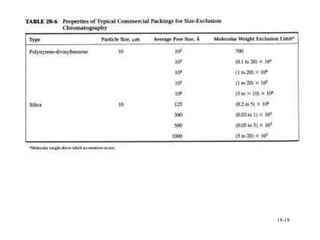
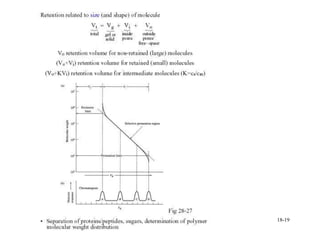
This document discusses gas chromatography and its principles and instrumentation. It covers topics like vaporization of samples, retention volumes, common carrier gases, types of columns, and popular detectors like the flame ionization detector, thermal conductivity detector, and electron capture detector. It also discusses high performance liquid chromatography, the different modes like partition chromatography, and applications like gel permeation size exclusion chromatography.