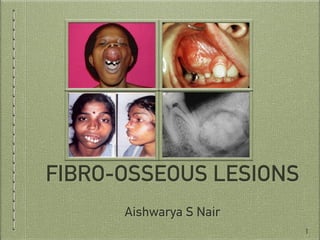
This document provides information on fibro-osseous lesions, with a focus on fibrous dysplasia. It discusses the classification of fibro-osseous lesions and the importance of radiology in diagnosis. Regarding fibrous dysplasia, it describes the pathogenesis, classification into monostotic and polyostotic forms, and clinical features such as presentations in the jaw, skin pigmentation abnormalities, and craniofacial involvement. Radiographic features include mixed radiolucent-radiopaque appearances and deformities resulting from bone involvement.