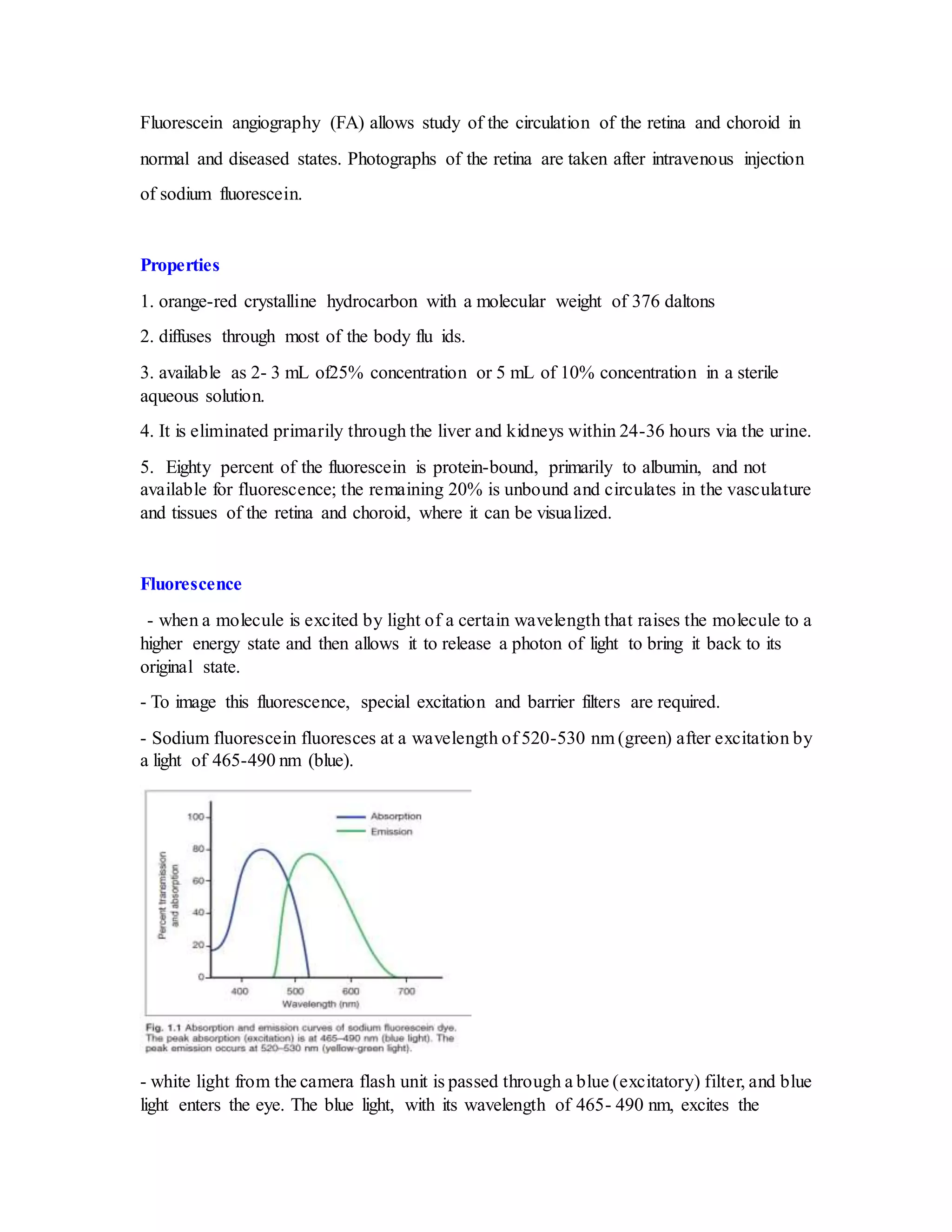
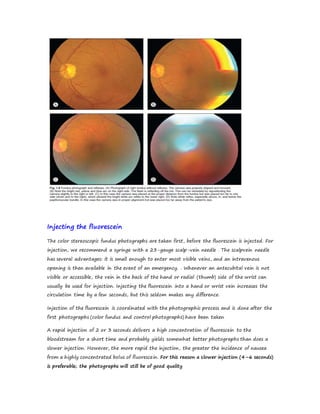
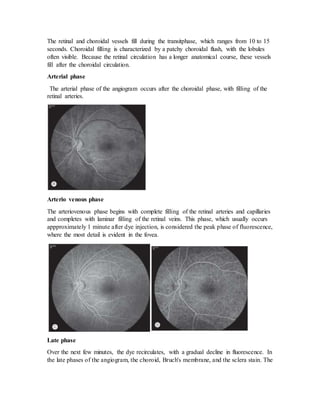
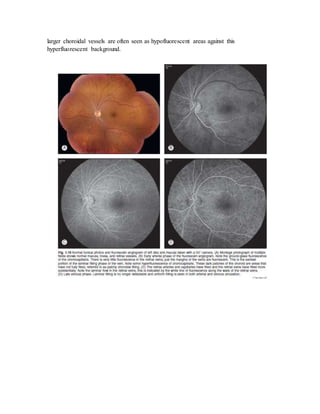
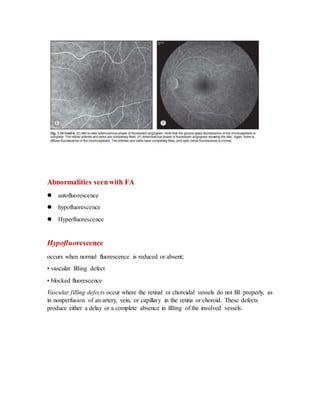
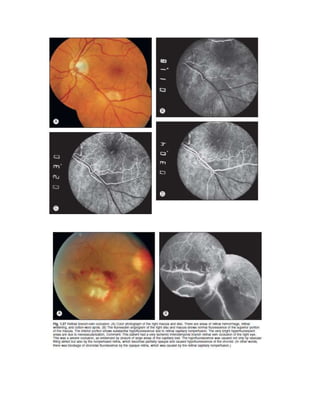
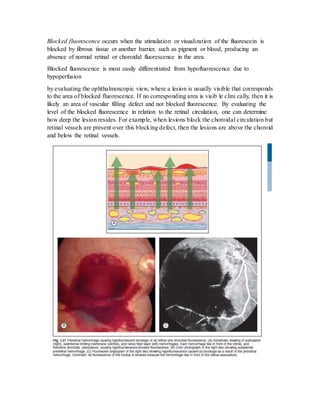
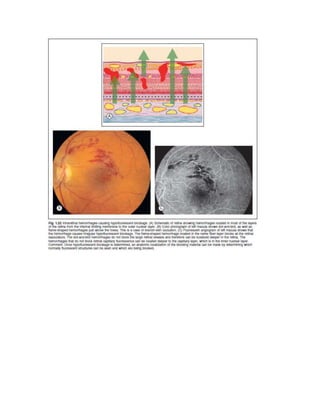
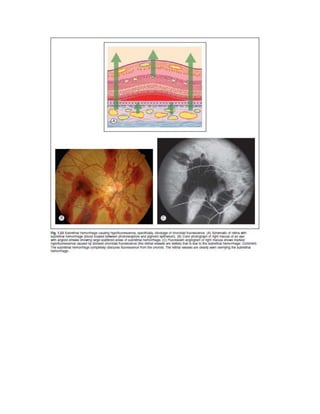
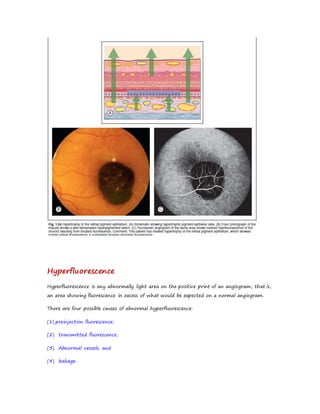
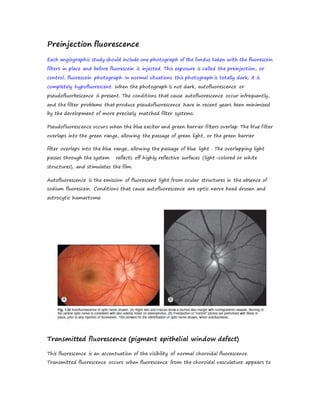
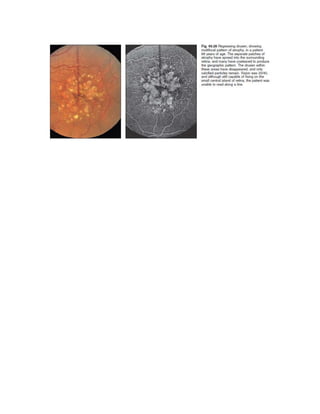
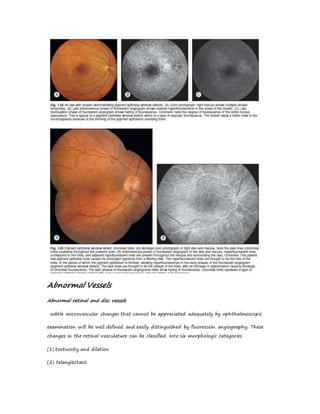
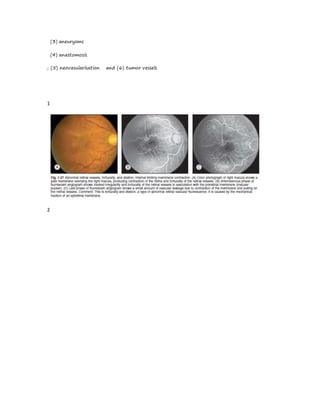
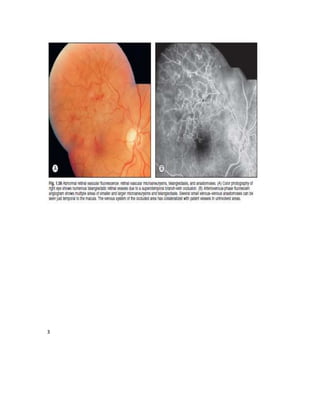
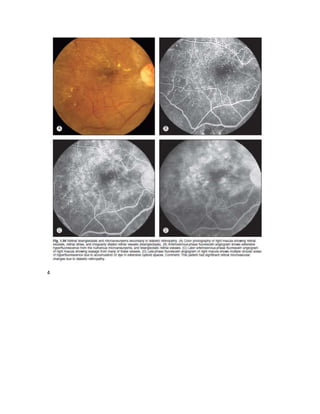
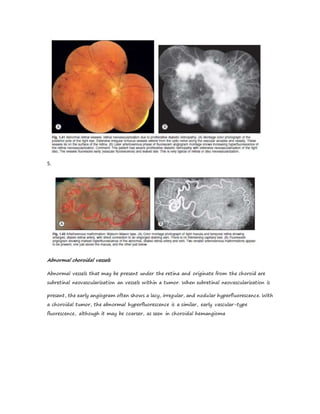
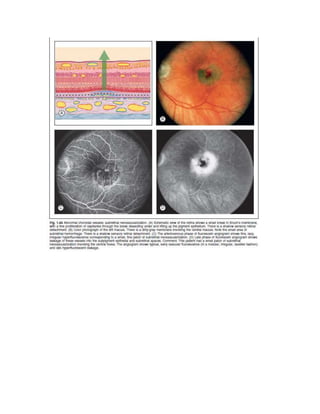
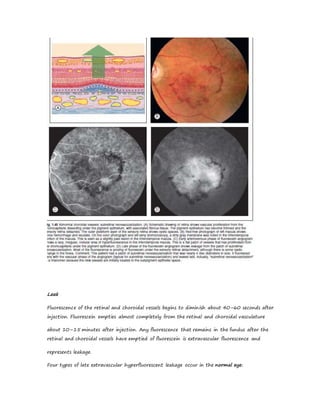
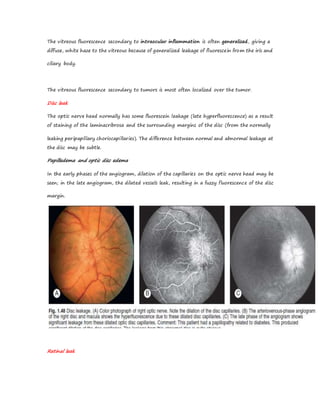
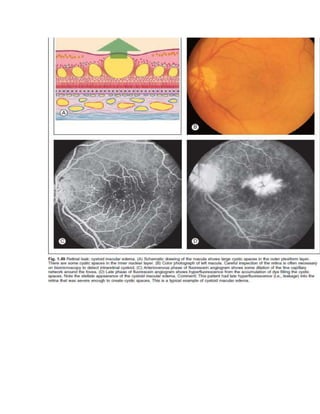
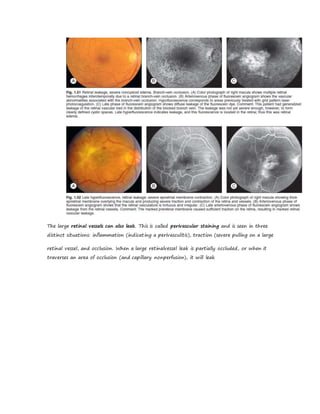
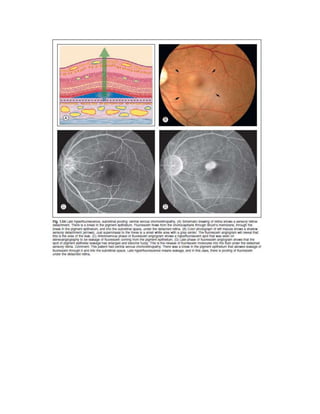
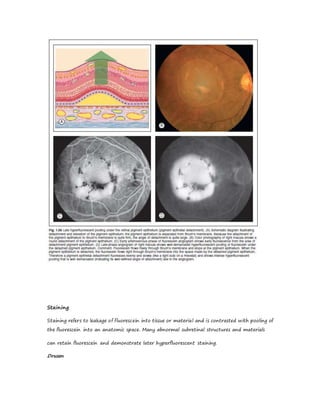
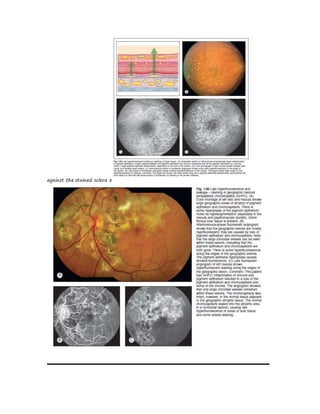
Fluorescein angiography allows visualization of the retina and choroid circulation by taking photographs after injecting sodium fluorescein dye intravenously. The dye circulates through the blood vessels and leaks through defective barriers, appearing as areas of hyperfluorescence on angiograms. Precise timing of photographs captures the early transit phase when vessels fill, followed by later phases showing normal and abnormal leakage patterns that help diagnose retinal conditions. Careful technique is required to safely perform high quality fluorescein angiography.