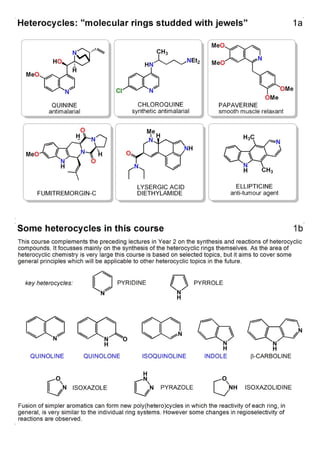
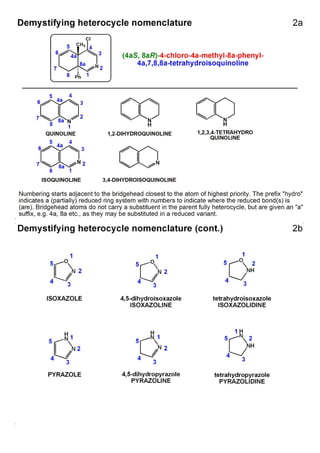
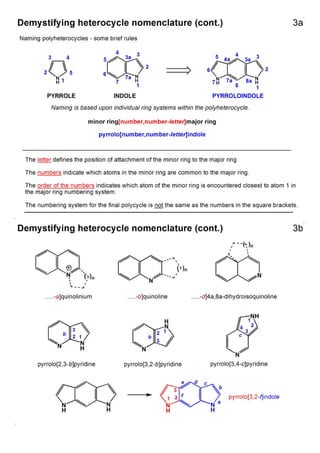
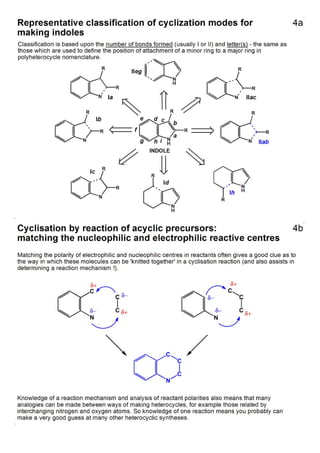
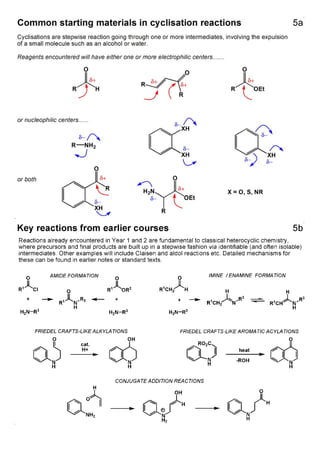
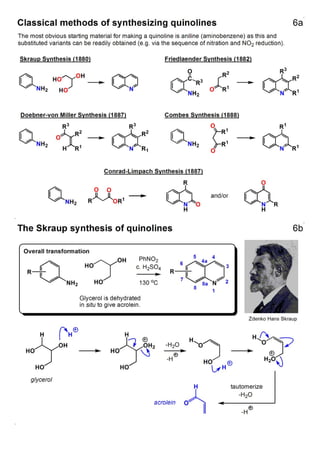
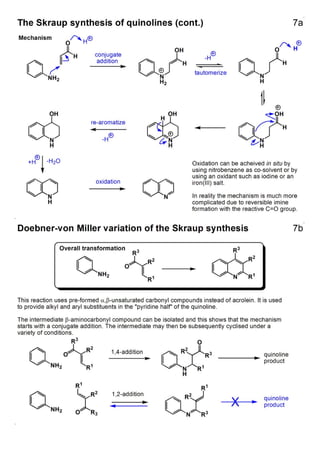
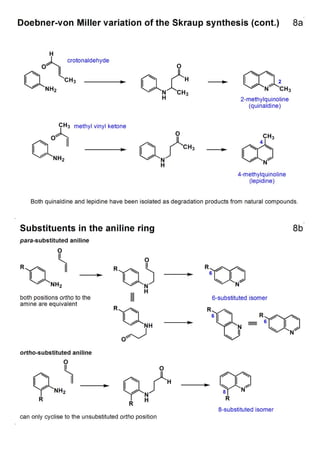
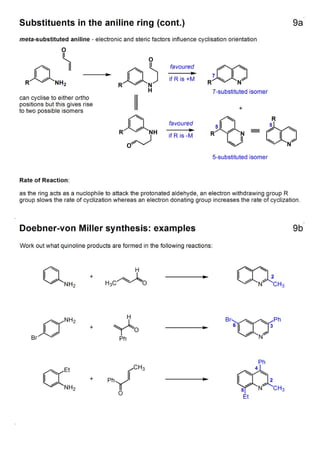
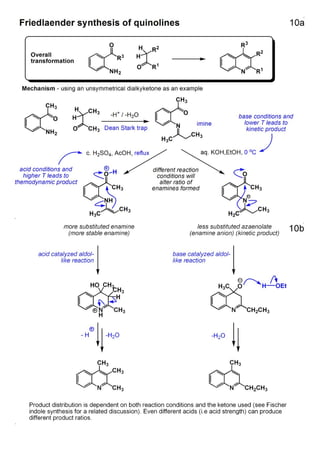
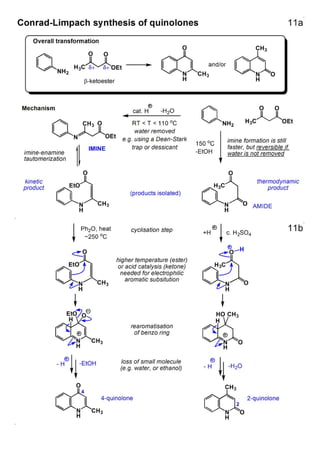
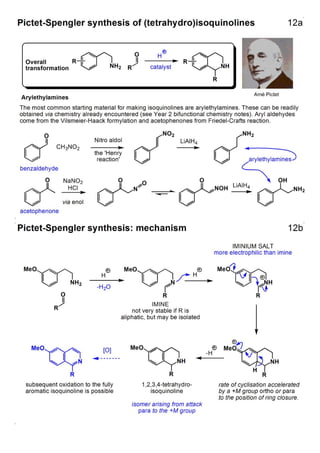
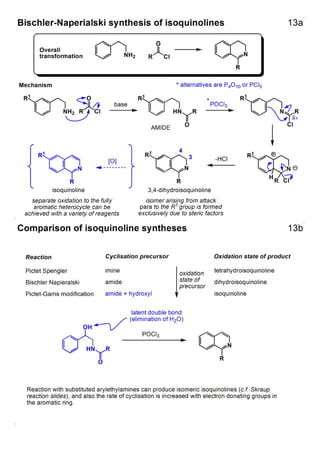
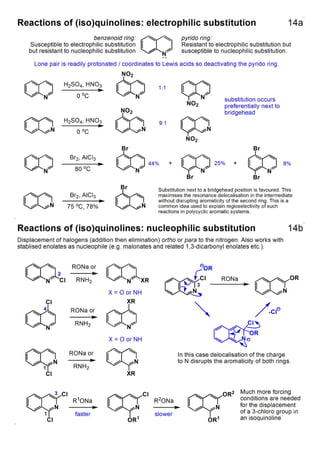
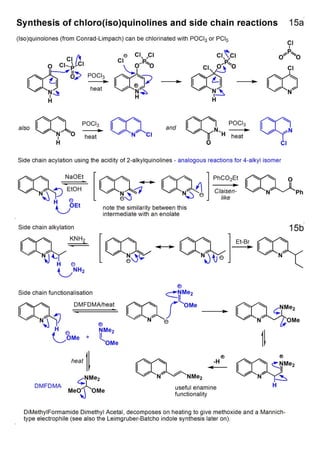
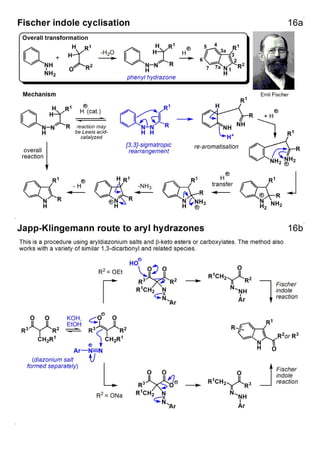
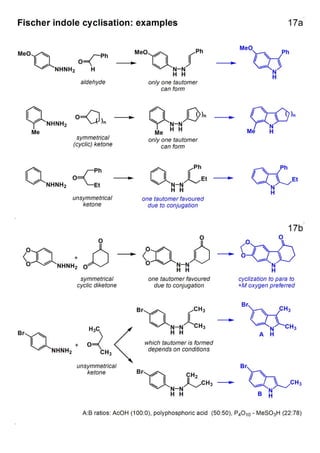
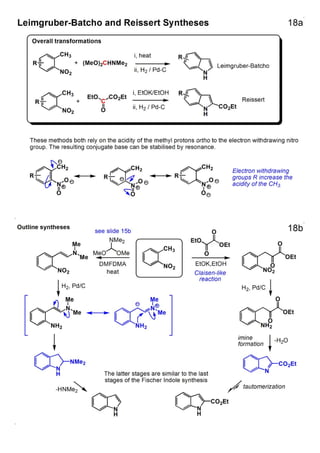
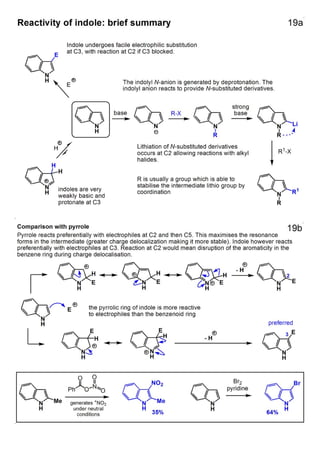
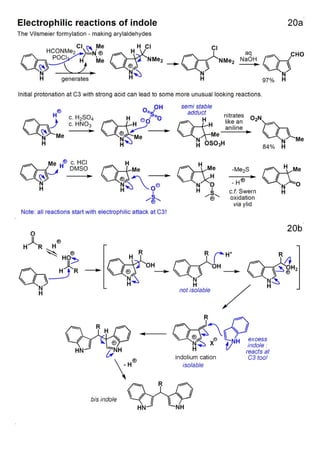
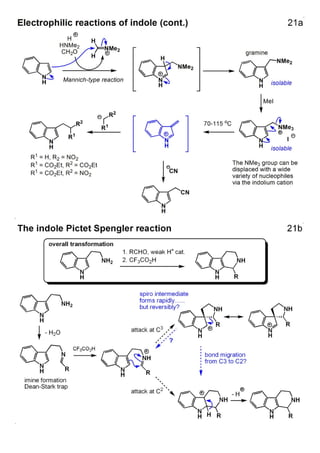
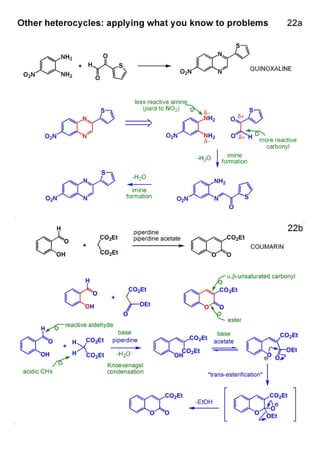
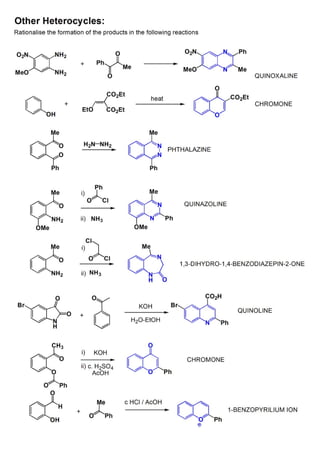
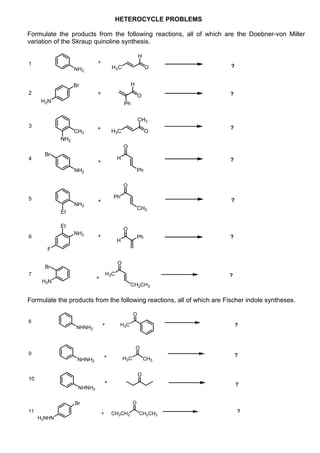
1. The document outlines the contents and learning outcomes of a course on heterocyclic chemistry in two parts. Part 1 covers the introduction and structures of various heterocycles. Part 2 focuses on 1,3-dipolar cycloaddition reactions and specific heterocycles like isoxazoles.
2. Students will learn to draw and name heterocycles, distinguish reaction types, predict products, apply reaction mechanisms, draw synthesis sequences, and explain isomer distributions.
3. Suggested reading materials and online notes are provided to aid student learning and practice of sample exam questions.