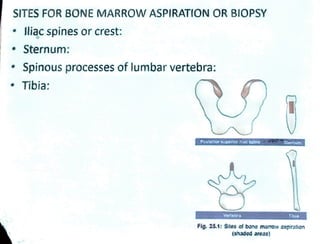
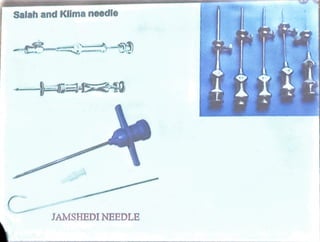
Bone marrow is located within bone cavities and consists of hematopoietic cells, sinusoids, fibroblasts, fat cells, and macrophages. It is the site of blood cell production. There are two types of marrow - red, which is active hematopoietic tissue, and yellow, which comprises inactive fatty tissue. Bone marrow aspiration and biopsy are techniques used to sample marrow. Aspiration uses needles to extract a small amount of fluid marrow without damage to bone, while biopsy extracts a core of marrow and bone using a larger needle. Examination of bone marrow samples provides information about cell morphology, counts, architecture, and pathology and is indicated to investigate causes of blood abnormalities or suspected blood disorders.