- Sílvia Simon, Jordi Poater, Miquel Duran, and Miquel Solà from the Institut de Química Computaciona i Catàlisi, Universitat de Girona presented on using everyday analogies to understand aromaticity.
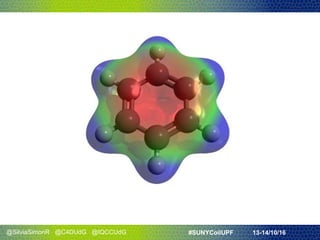
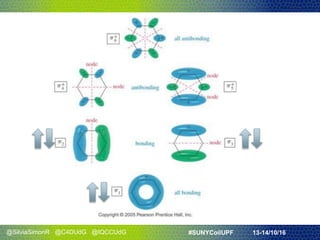
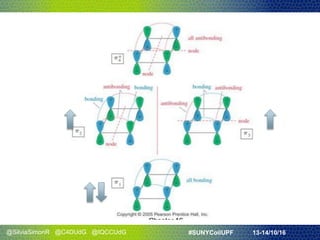
- They discussed Hückel's rule for aromaticity in monocyclic systems with planar symmetry and how it strictly applies to systems with 4n+2 electrons in the ring.
- The presentation concluded that everyday analogies can help explain aromaticity if combined with visual tools, and concepts need ways for people to see, touch, and experience them.