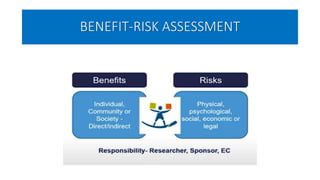
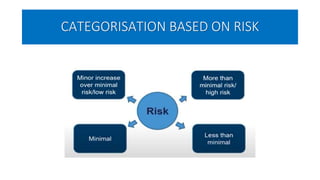
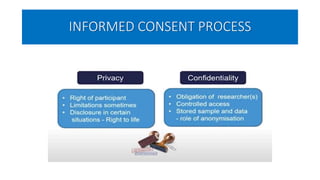
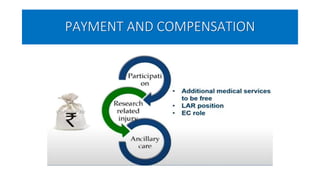
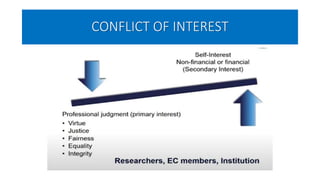
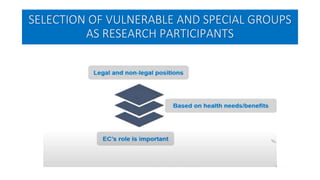
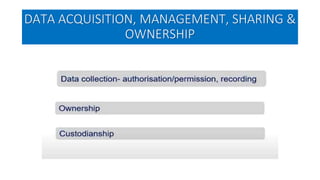
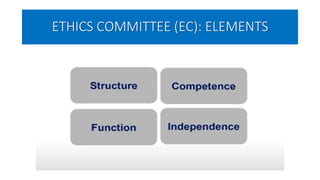
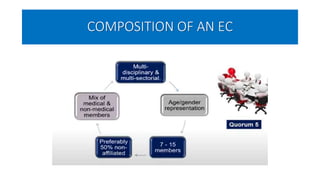
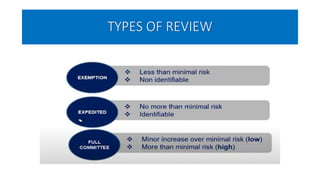
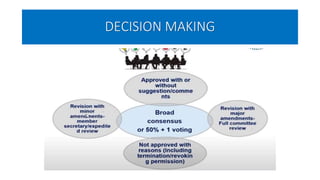
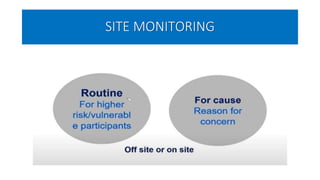
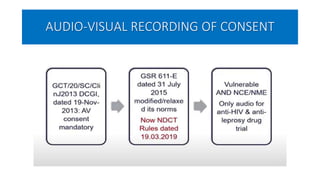
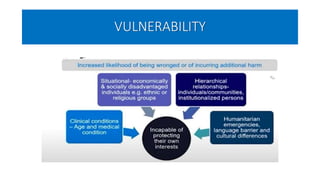
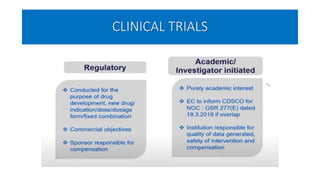
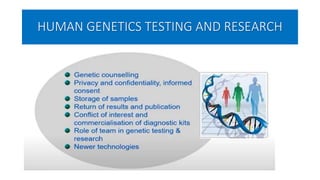
This document summarizes the National Ethical Guidelines for Biomedical and Health Research Involving Human Participants released by the Indian Council of Medical Research (ICMR) in 2017. The guidelines cover 12 sections that establish general ethical principles, review procedures, informed consent processes, and regulations for research involving vulnerable groups or children. Key points include requirements for ethics committee review and clinical trial registration, guidelines for informed consent depending on the situation, ownership of biological materials and data, and conducting research during emergencies or with vulnerable populations.