This document outlines engineering assumptions and equations for analyzing energy conversion cycles that use air as the working fluid. Key assumptions include treating air as a single species with constant specific heat, and using the ideal gas law. Equations presented include the continuity, momentum and energy conservation equations, as well as equations of state and equations specific to analyzing cycles like Otto, Brayton, and Diesel cycles. Thermodynamic property data for air is also referenced.


![Basic Conservation Equations
Continuity Equation
m = ρvA [kg/s]
Momentum Equation
F = (vm + pA)out - in [N]
Energy Equation
Q - W = ((h + v2/2 + gh)m)out - in [kW]
Basic Engineering Equations](https://image.slidesharecdn.com/ec-equations-180214042656/85/Engineering-Energy-Conversion-Assumptions-and-Equations-3-320.jpg)
![Ideal Gas State Equation
pv = RT [kJ/kg]
Perfect Gas
cp = constant [kJ/kg*K]
Kappa
χ = cp/cv [/]
For air: χ = 1.4 [/], R = 0.2867 [kJ/kg*K] and
cp = 1.004 [kJ/kg*K]
Basic Engineering Equations](https://image.slidesharecdn.com/ec-equations-180214042656/85/Engineering-Energy-Conversion-Assumptions-and-Equations-4-320.jpg)
![Power Cycles Engineering Equations
Carnot Cycle Efficiency
= 1 - TR/TA
Otto Cycle Efficiency
= 1 - 1/ε(χ-1)
Brayton Cycle Efficiency
= 1 - 1/rp
(χ-1)/χ
Diesel Cycle Efficiency
= 1 - (φχ-1)/ (χε(χ-1)(φ-1))
Cycle Efficiency
= Wnet/Q [/]
Heat Rate
HR = (1/)3,412 [Btu/kWh]
rp = p2/p1 [/]; ε = V1/V2 [/]; φ = V3/V2 [/]](https://image.slidesharecdn.com/ec-equations-180214042656/85/Engineering-Energy-Conversion-Assumptions-and-Equations-5-320.jpg)
![Power Cycles Engineering Equations
Otto Cycle
wnet = qh - ql = cv(T3 - T2) - cv(T4 - T1) [kJ/kg]
Wnet = wnetm [kW]
Brayton Cycle
wnet = qh - ql = cp(T3 - T2) - cp(T4 - T1) [kJ/kg]
Wnet = wnetm [kW]
Diesel Cycle
wnet = qh - ql = cp(T3 - T2) - cv(T4 - T1) [kJ/kg]
Wnet = wnetm [kW]](https://image.slidesharecdn.com/ec-equations-180214042656/85/Engineering-Energy-Conversion-Assumptions-and-Equations-6-320.jpg)
![Isentropic Compression
T2/T1 = (p2/p1)(χ-1)/χ [/]
T2/T1 = (V1/V2)(χ-1) [/]
p2/p1 = (V1/V2)χ [/]
wc = cp(T2 - T1) [kJ/kg]
Wc = cp(T2 - T1)m [kW]
Power Cycle Components/Processes
Engineering Equations](https://image.slidesharecdn.com/ec-equations-180214042656/85/Engineering-Energy-Conversion-Assumptions-and-Equations-7-320.jpg)
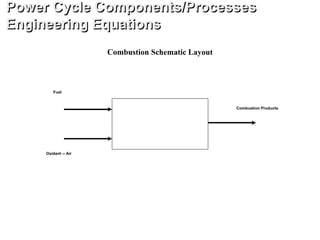
![Combustion is ideal, complete with no heat loss and at
stoichiometric conditions.
Also,
Flame Temperature [K]
hreactants = hproducts [kJ/kg]
Power Cycle Components/Processes
Engineering Equations](https://image.slidesharecdn.com/ec-equations-180214042656/85/Engineering-Energy-Conversion-Assumptions-and-Equations-8-320.jpg)
![Specific Enthalpy vs Temperature
-20,000
-10,000
0
10,000
20,000
30,000
40,000
50,000
60,000
70,000
80,000
90,000
500 800 1,100 1,400 1,700 2,000 2,300 2,600 2,900 3,200 3,500 3,800 4,100 4,400 4,700 5,000
C(S) H2 S(S) N2 O2 H2O(L) CH4 CO2 H2O SO2
SpecificEnthalpy[kJ/kg]
Temperature [K]
Power Cycle Components/Processes
Engineering Equations](https://image.slidesharecdn.com/ec-equations-180214042656/85/Engineering-Energy-Conversion-Assumptions-and-Equations-10-320.jpg)
![Combustion h – T Diagram
SpecificEnthalpy--h[kJ/kg]
Temperature -- T [K]
Reactants
Products
TflameTreference
Power Cycle Components/Processes
Engineering Equations](https://image.slidesharecdn.com/ec-equations-180214042656/85/Engineering-Energy-Conversion-Assumptions-and-Equations-11-320.jpg)
![Isentropic Expansion
T1/T2 = (p1/p2)(χ-1)/χ [/]
T1/T2 = (V2/V1)(χ-1) [/]
p1/p2 = (V2/V1)χ [/]
we = cp(T1 - T2) [kJ/kg]
We = cp(T1 - T2)m [kW]
Power Cycle Components/Processes
Engineering Equations](https://image.slidesharecdn.com/ec-equations-180214042656/85/Engineering-Energy-Conversion-Assumptions-and-Equations-12-320.jpg)
![Sonic Velocity
vs = (χ RT)1/2 [m/s]
Mach Number
M = v/vs [/]
Compressible Flow Engineering Equations](https://image.slidesharecdn.com/ec-equations-180214042656/85/Engineering-Energy-Conversion-Assumptions-and-Equations-13-320.jpg)
![Isentropic Flow
Tt/T = (1 + M2(χ - 1)/2) [/]
pt/p = (1 + M2(χ - 1)/2)χ/(χ-1) [/]
ht = (h + v2/2) [kJ/kg]
Tt = (T + v2/(2cp)) [K]
Thrust = vm + (p - pa)A [N]
Compressible Flow Engineering Equations](https://image.slidesharecdn.com/ec-equations-180214042656/85/Engineering-Energy-Conversion-Assumptions-and-Equations-14-320.jpg)