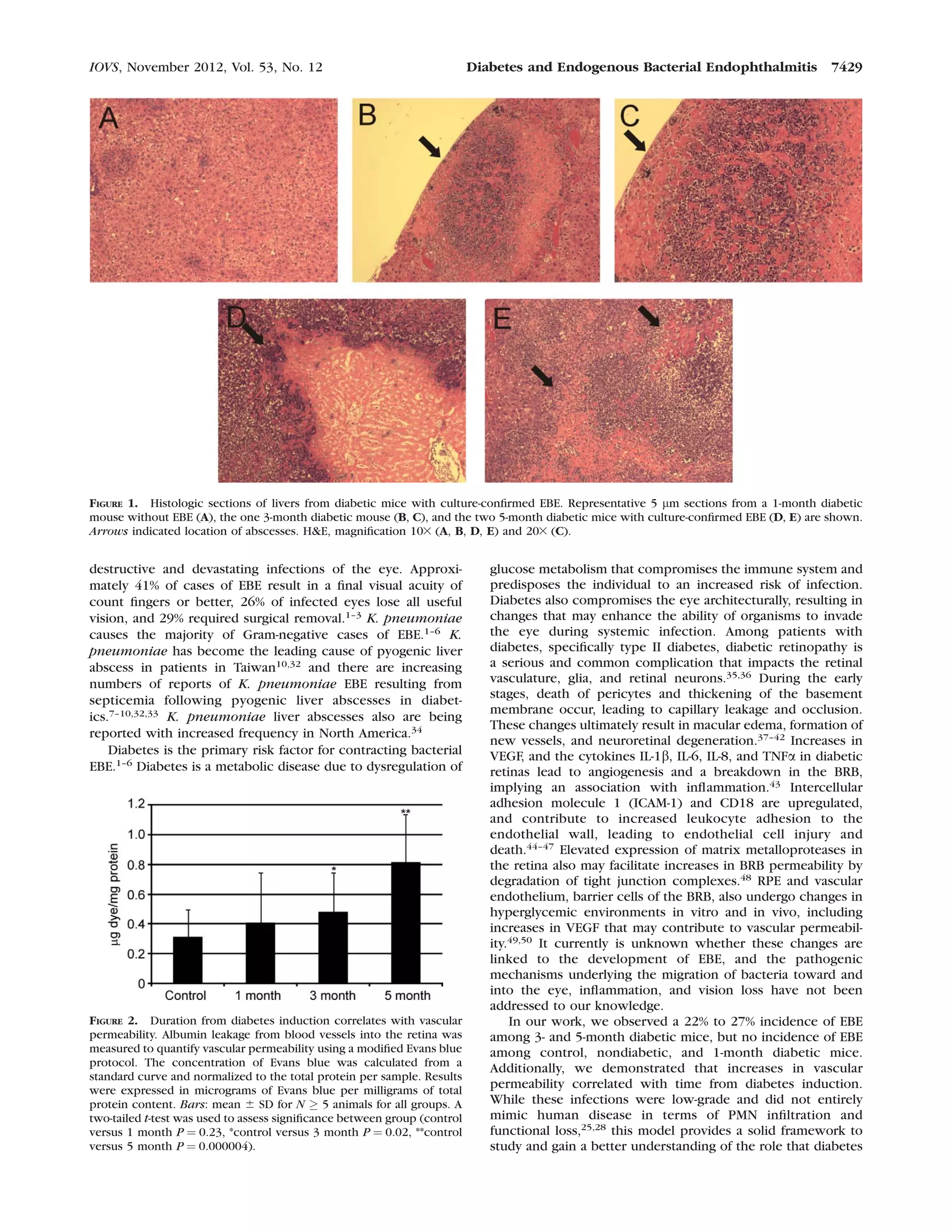
This document discusses a study that tested the hypothesis that changes in the diabetic ocular environment facilitate the development of endogenous bacterial endophthalmitis (EBE). Mice were rendered diabetic for varying durations (1, 3, or 5 months) using streptozotocin injections. Diabetic and non-diabetic mice were then infected with Klebsiella pneumoniae via tail vein injection. Results showed that longer diabetes duration (3-5 months) correlated with higher EBE incidence and greater blood-retinal barrier permeability, supporting the hypothesis that diabetic ocular changes contribute to EBE development.