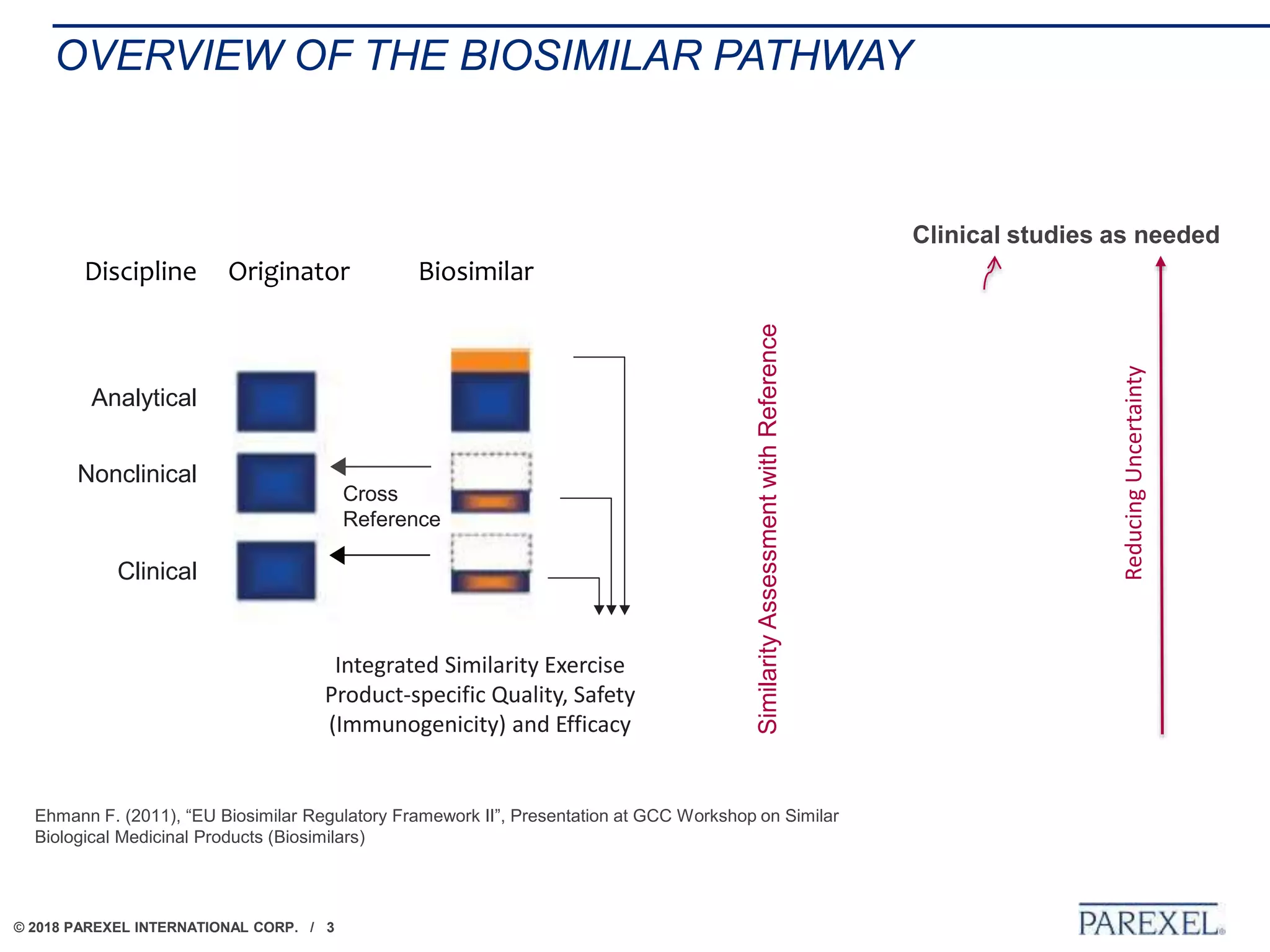
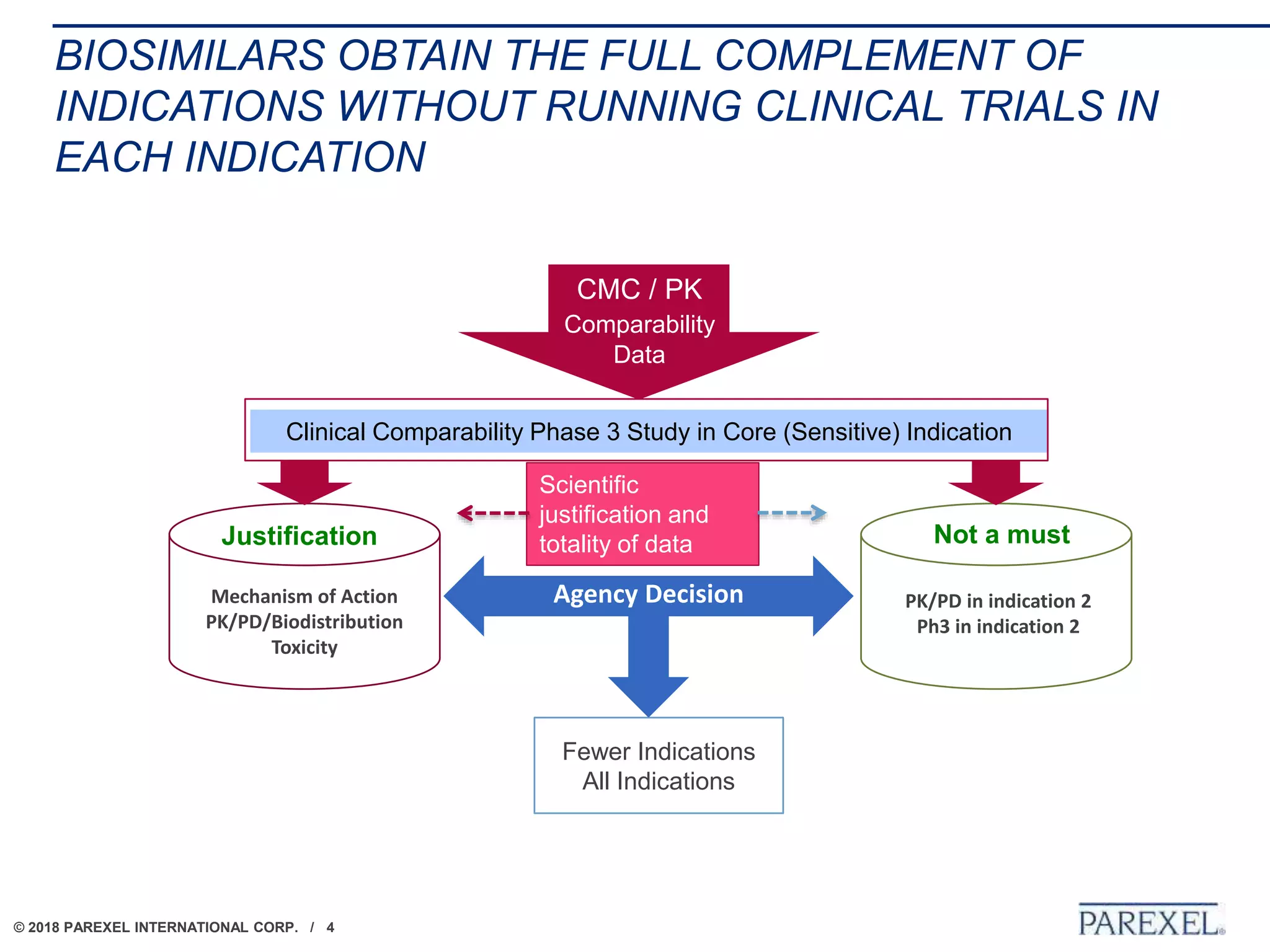
The document discusses the lifecycle and regulatory pathway for biosimilars, highlighting the FDA's 351(k) approval process, challenges post-approval including manufacturing changes and interchangeability issues, and the importance of maintaining quality and safety. It also addresses the varying definitions and regulations around interchangeability and switching among different jurisdictions, along with the complexities of managing post-approval changes. Lastly, the document notes ongoing challenges with biosimilar labeling and the need for collaboration in navigating these issues.











![© 2018 PAREXEL INTERNATIONAL CORP. / 14
IN EUROPE, DECISIONS REGARDING INTERCHANGEABILITY AND SWITCHING
ARE LEFT TO MEMBER STATES AND APPROACHES VARY
14©2015 NIBRT AbbVie | Biosimilars are not generics: Important considerations fueling the regulatory debate
UK
Recommends that biologic
prescriptions be written by
brand name in order to
“ensure that automatic
substitution of a biosimilar
product does not occur
when the medicine is
dispensed by the
pharmacist.”2
Germany
“[A]ny decision needs to be based on
scientific data, in particular data supporting a
high degree of comparability of the biosimilar
and its originator product and the scientific
plausibility of all data referred to in the
discussion process.”3
Norway
Automatic substitution currently
not allowed; government
sponsored “NOR-SWITCH” study
is ongoing; may be used to
support automatic substitution in
the future.
Ireland
“It is not recommended that
patients switch back and
forth between a biosimilar
and reference medicine, as
at the current time the
availability of data on the
impact of this are limited.”1
1. Ireland Health Products Regulatory Agency, Guide to Biosimilars for Healthcare Professionals and Patients, 14 October 2015. , 2. MHRA, Drug Safety Update:
Biosimilar products (February 2008), https://www.gov.uk/drug-safety-update/biosimilar-products. , 3. Paul Ehrlich Institute's position on the interchangeability of
biosimilars.](https://image.slidesharecdn.com/lifecyclebiosimilars2018final-180820144621/75/Life-of-a-Biosimilar-14-2048.jpg)