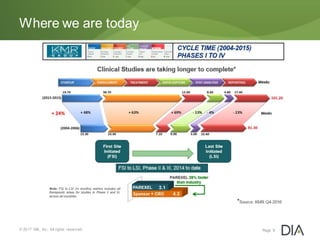
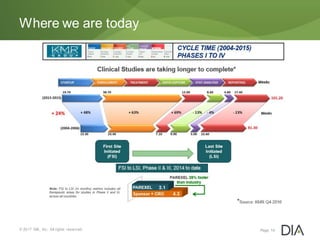
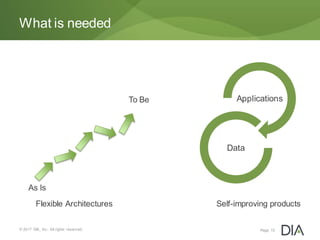
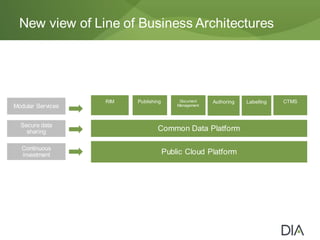
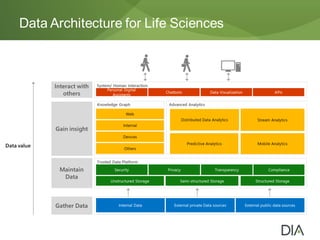
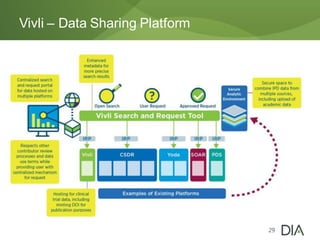
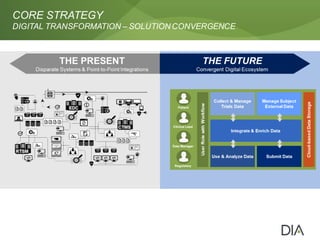
The document discusses the challenges in drug discovery and development, emphasizing that despite technological advancements, the time from discovery to approval is increasing due to inefficiencies in systems and processes. It highlights the necessity for innovative technologies, flexible architectures, and improved data integration to streamline regulatory submissions and enhance collaboration among organizations. Additionally, it underscores the importance of cloud-based solutions to facilitate these transitions and improve operational efficiency within the life sciences industry.