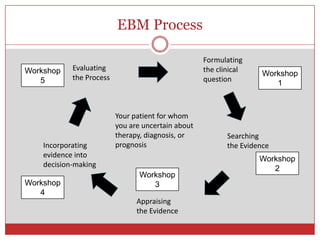
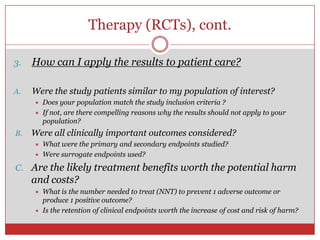
This document outlines the objectives and agenda for a workshop on journal clubs and evidence-based medicine reviews. The workshop will teach participants how to present clinical evidence-based medicine summaries to peers, critically appraise clinical studies, and discuss how to integrate evidence-based findings into clinical practice. Participants will have opportunities to present on their own clinical scenarios and evidence searches.