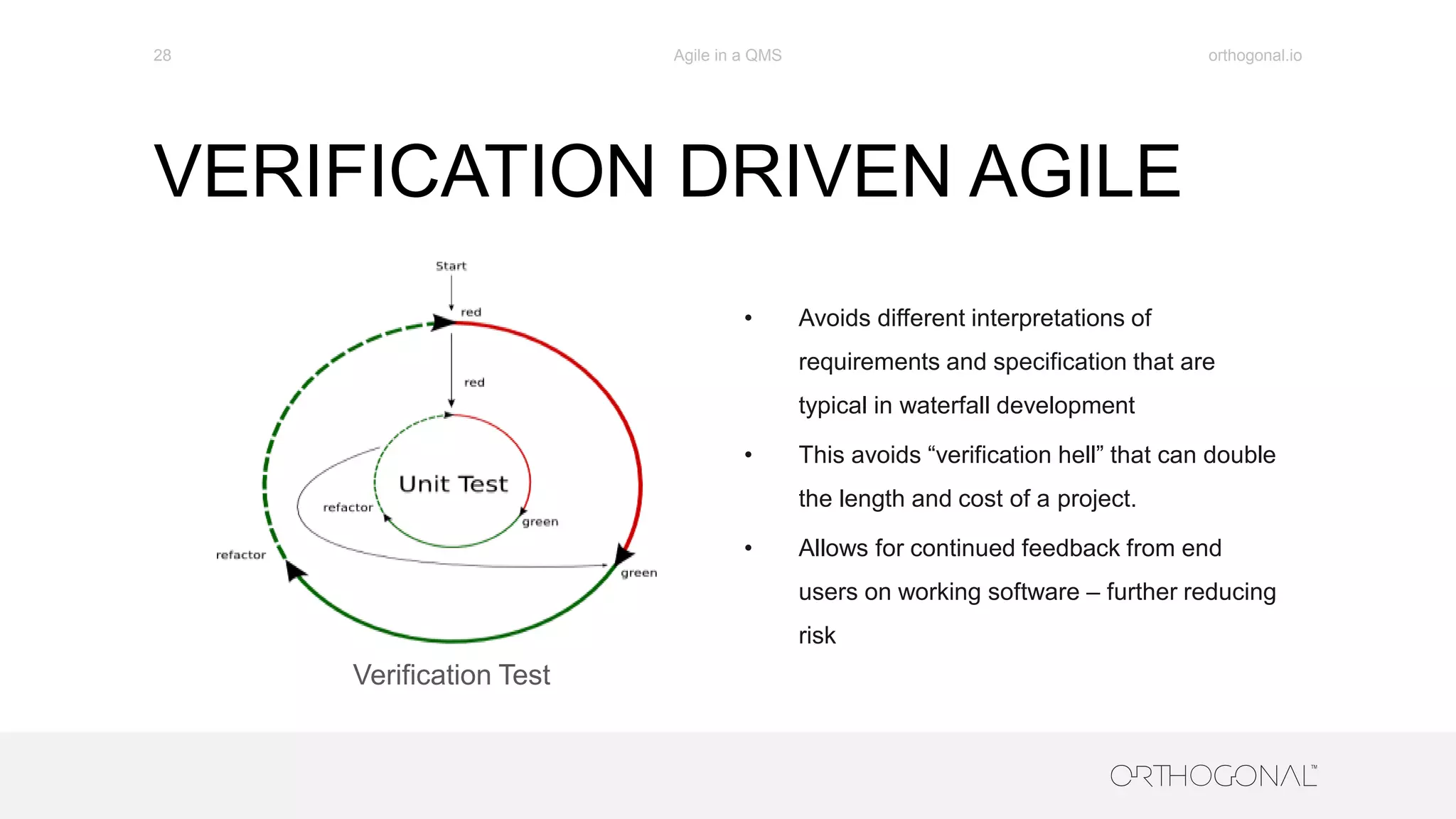
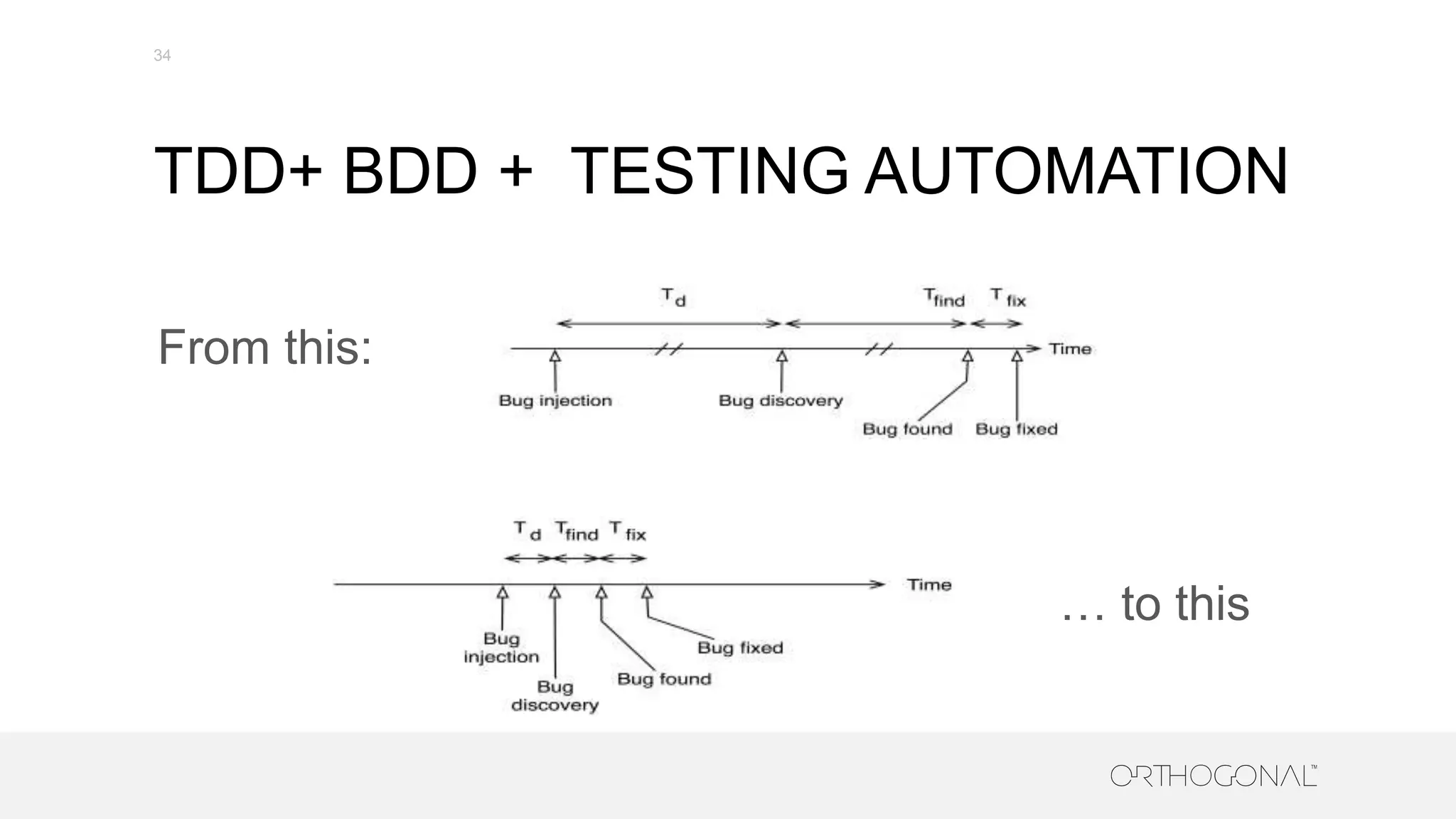
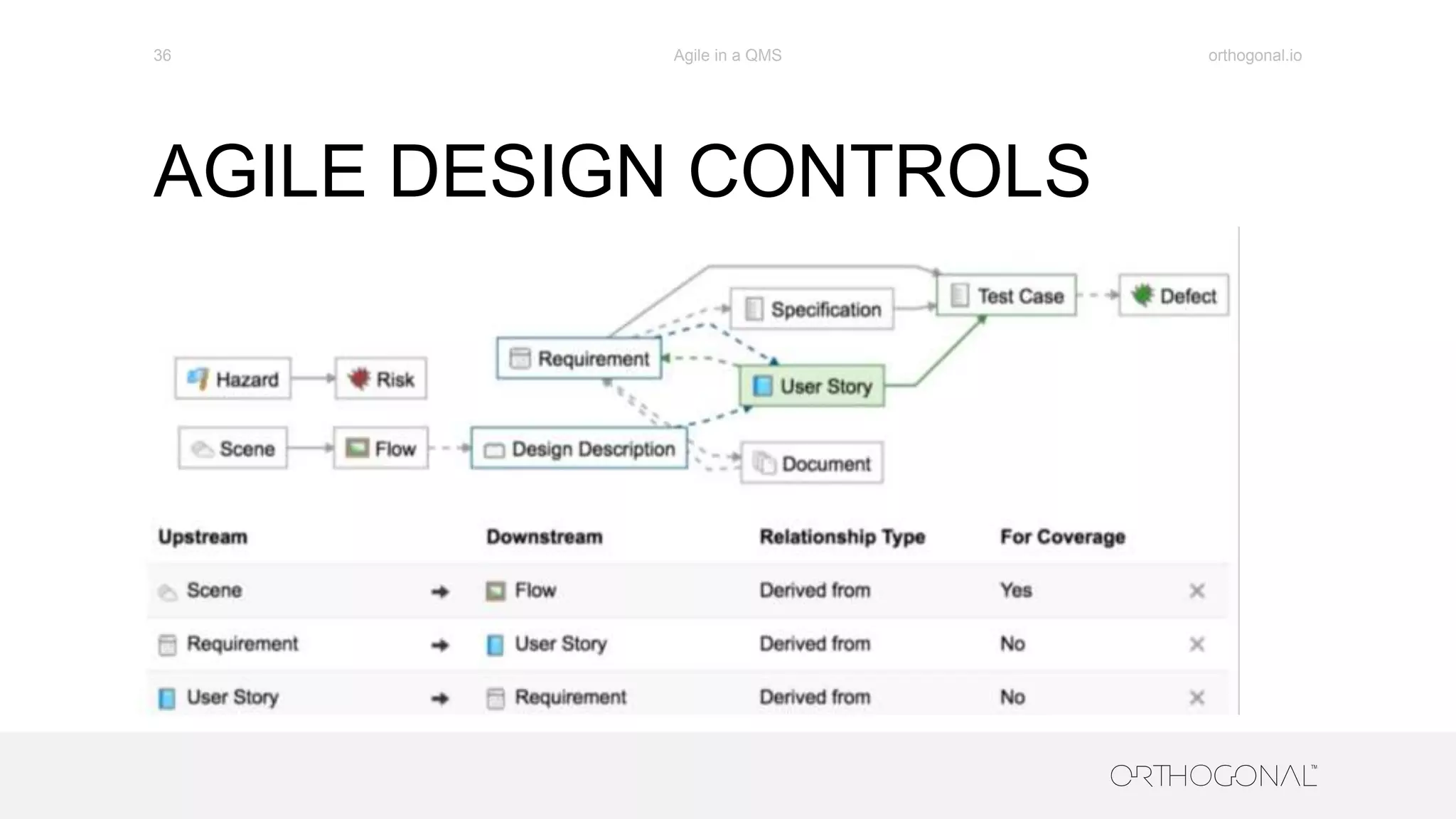
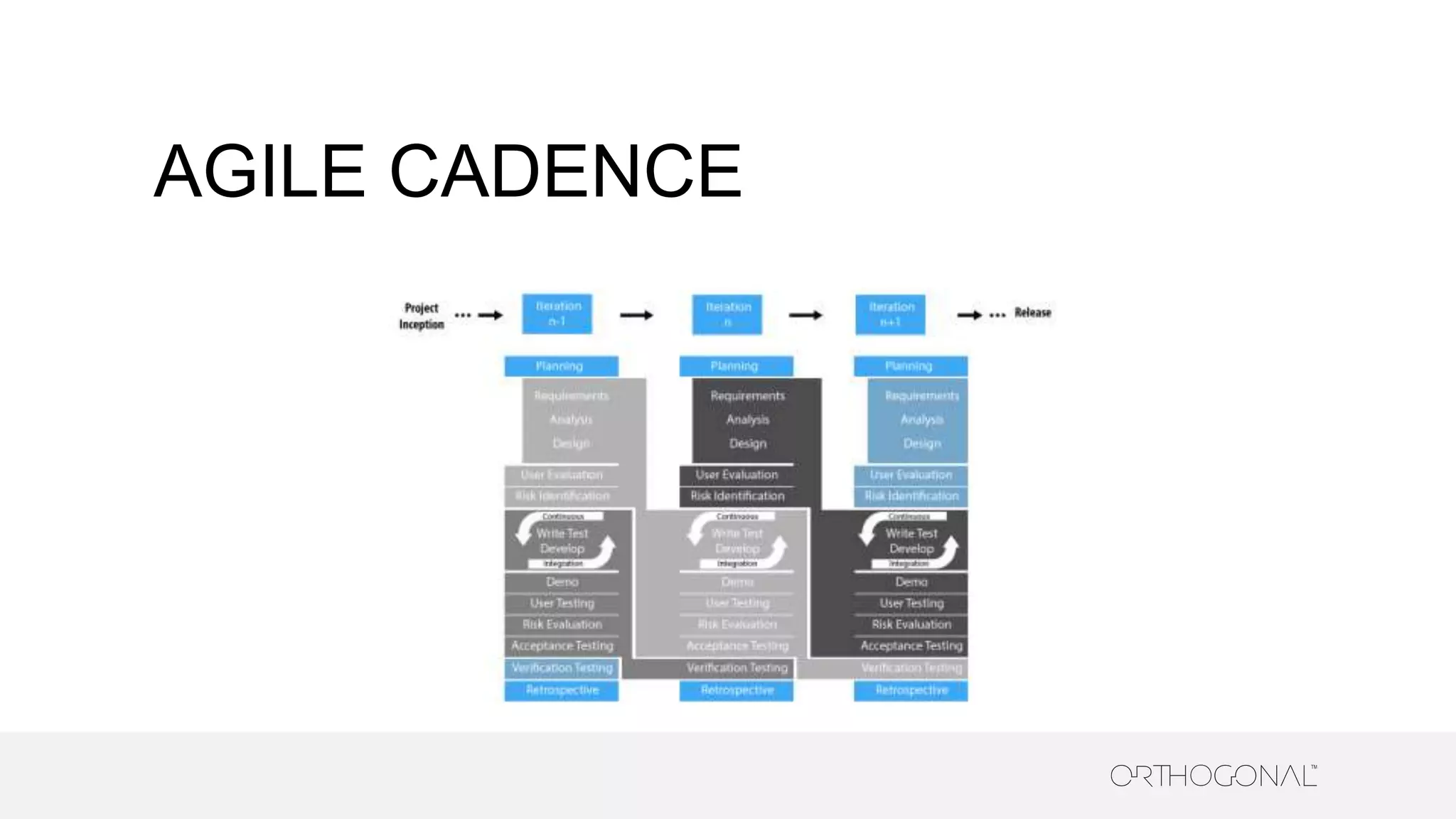
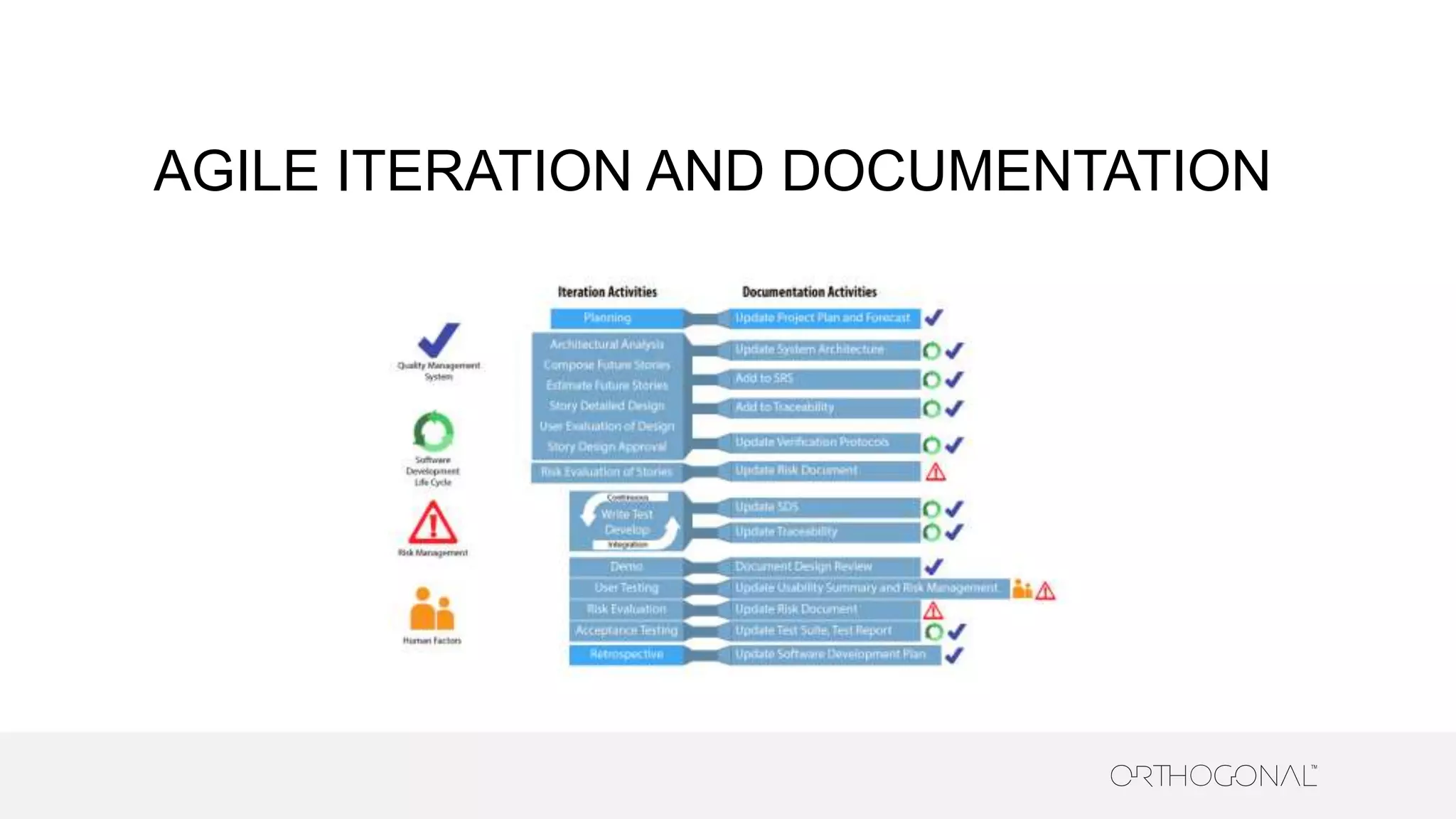
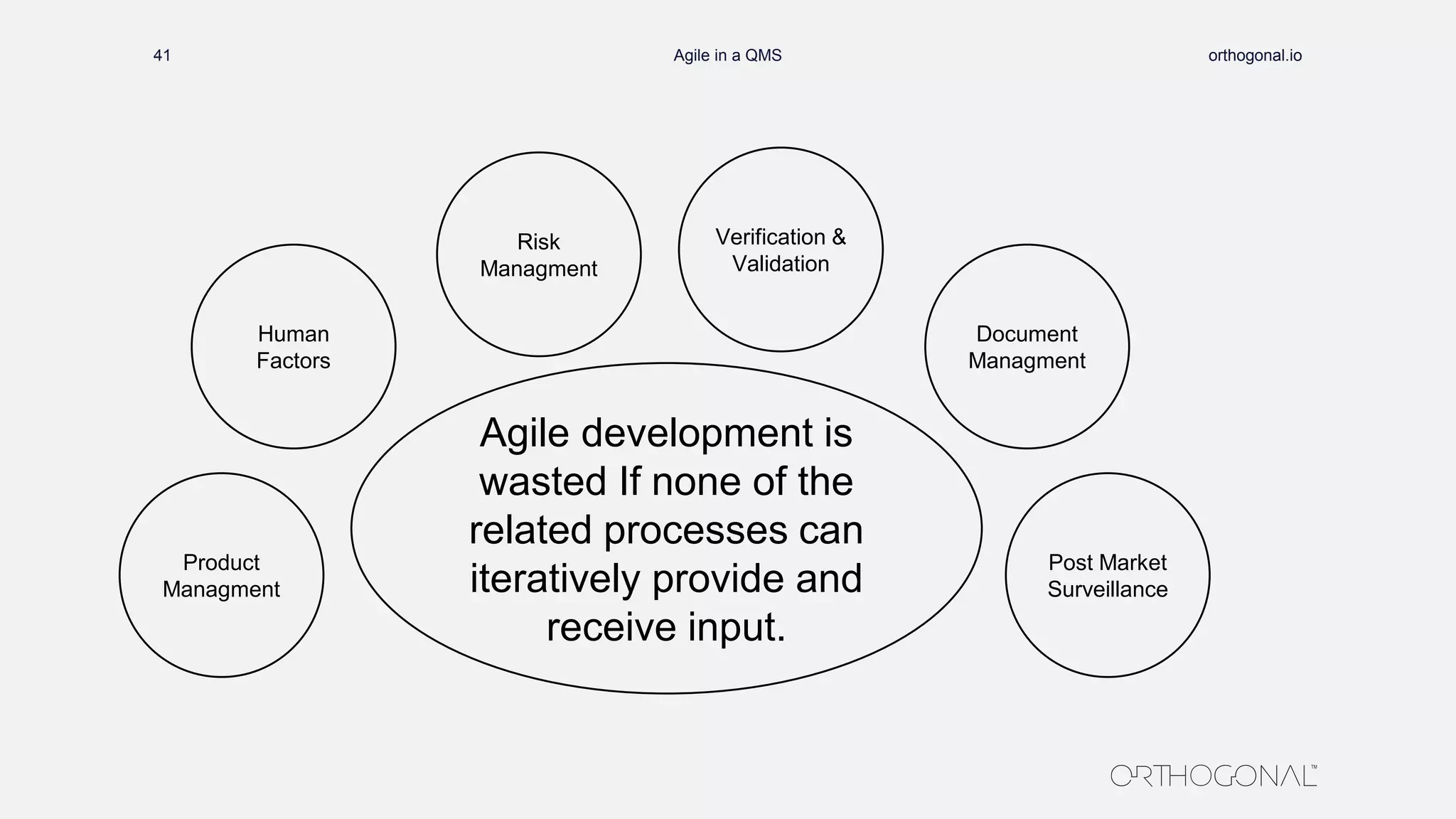
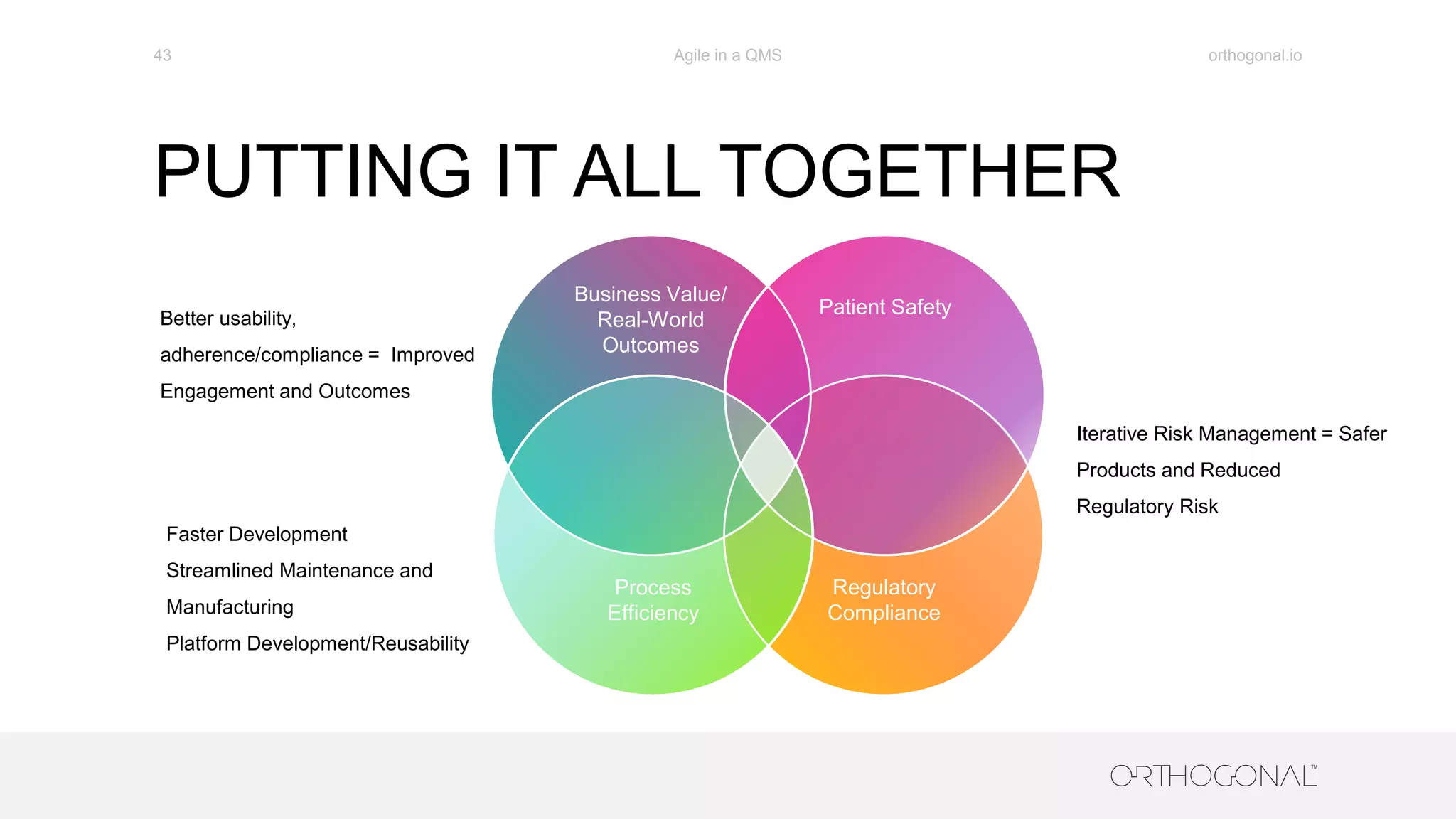
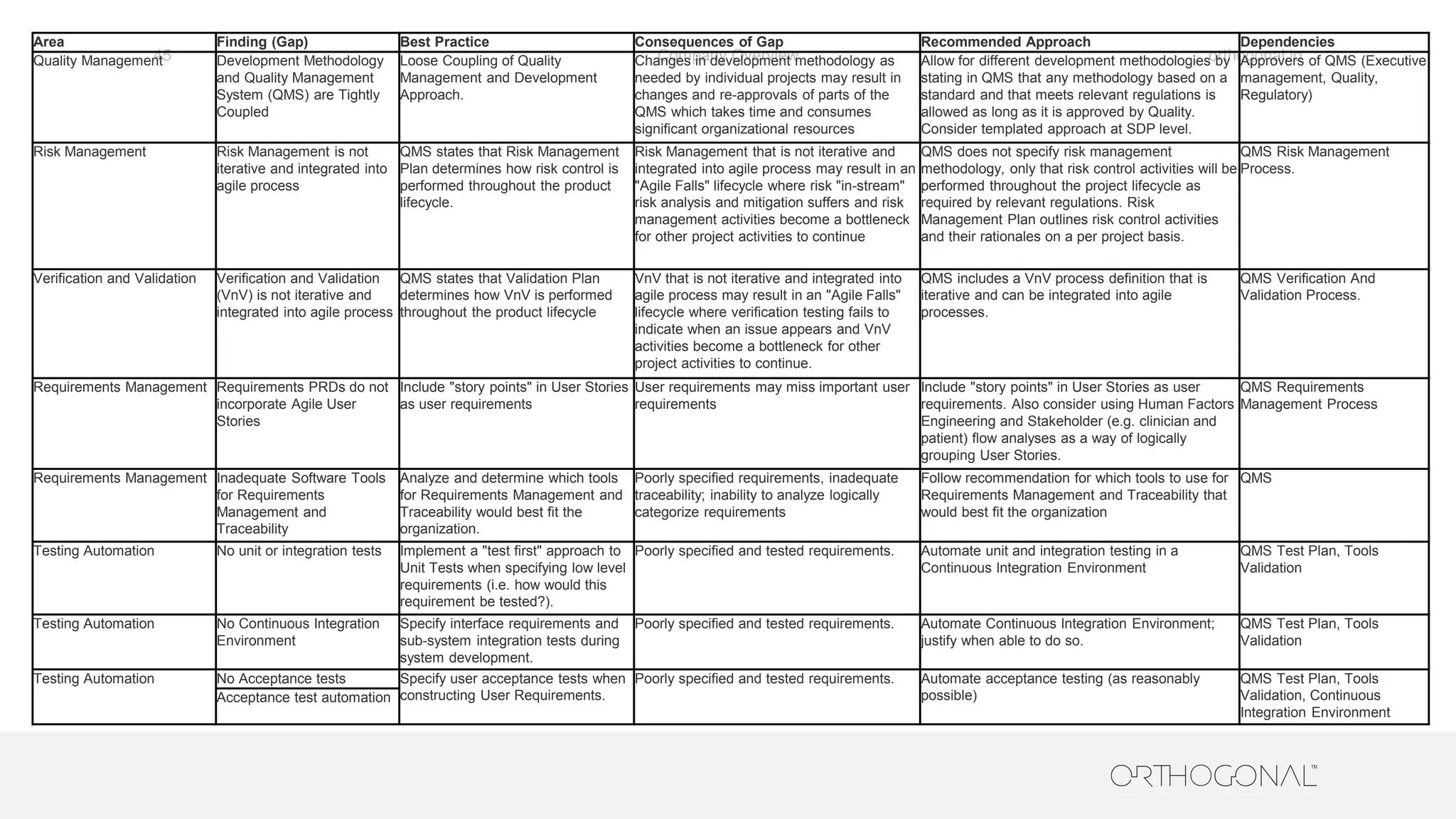
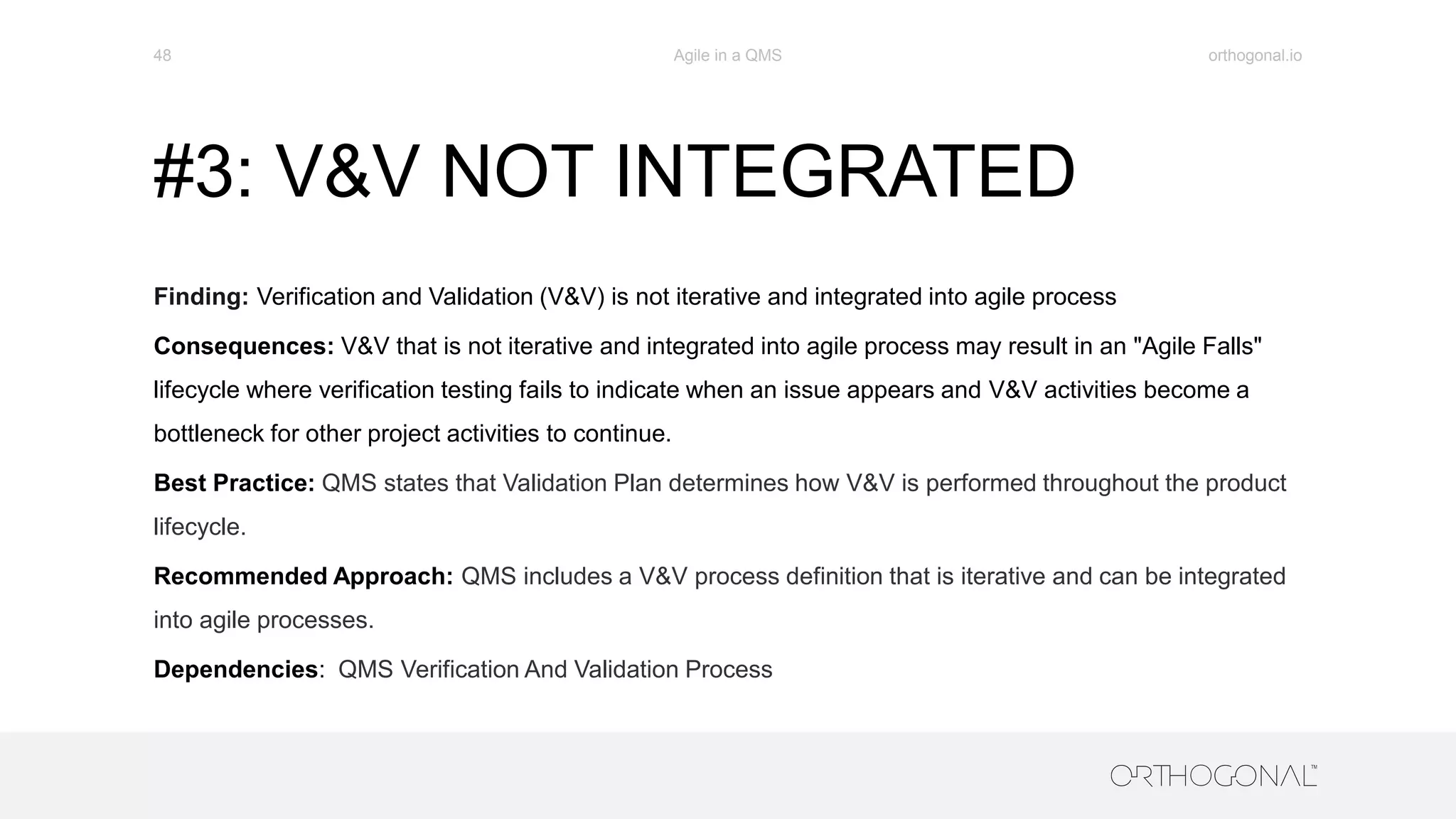
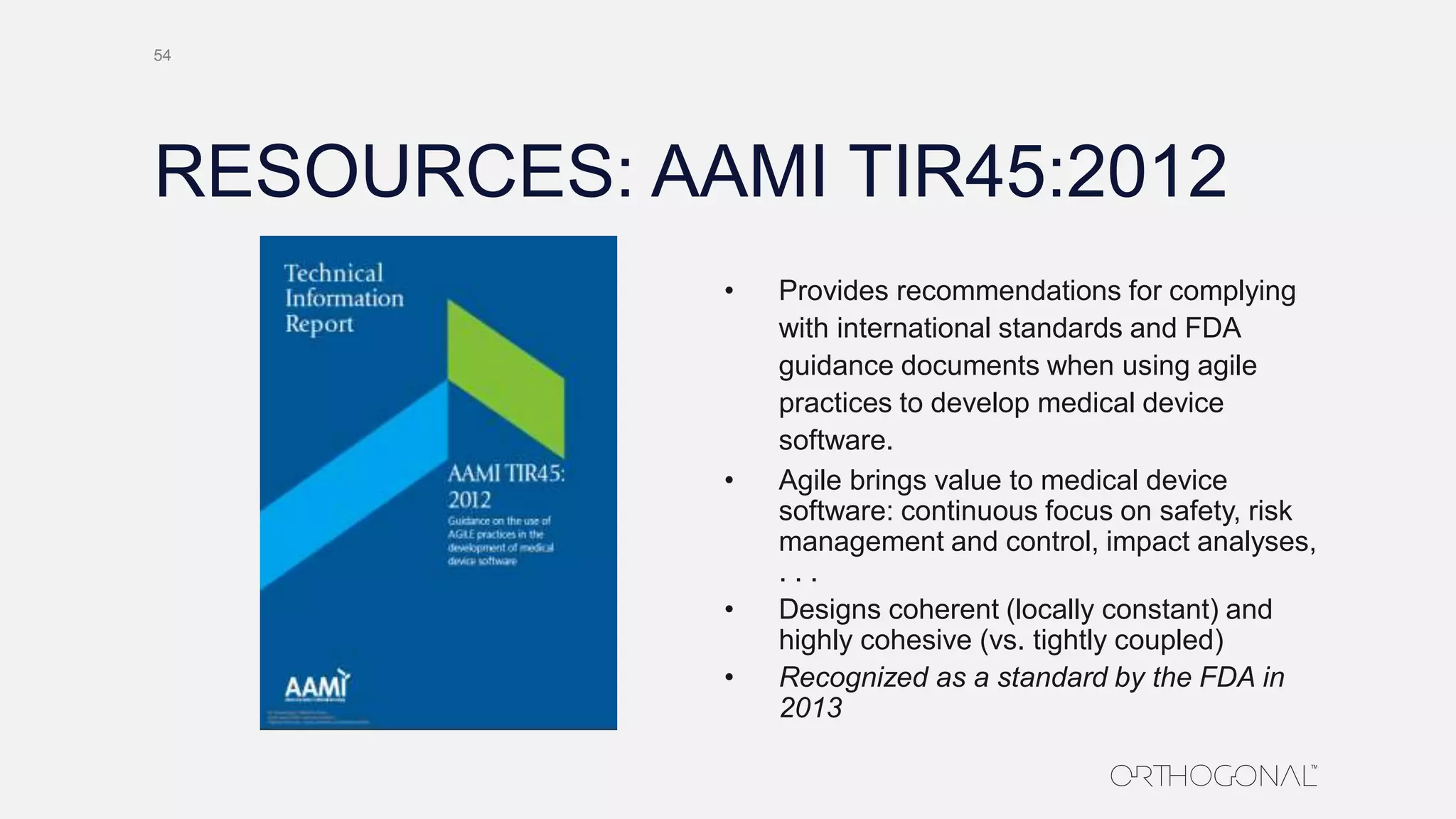
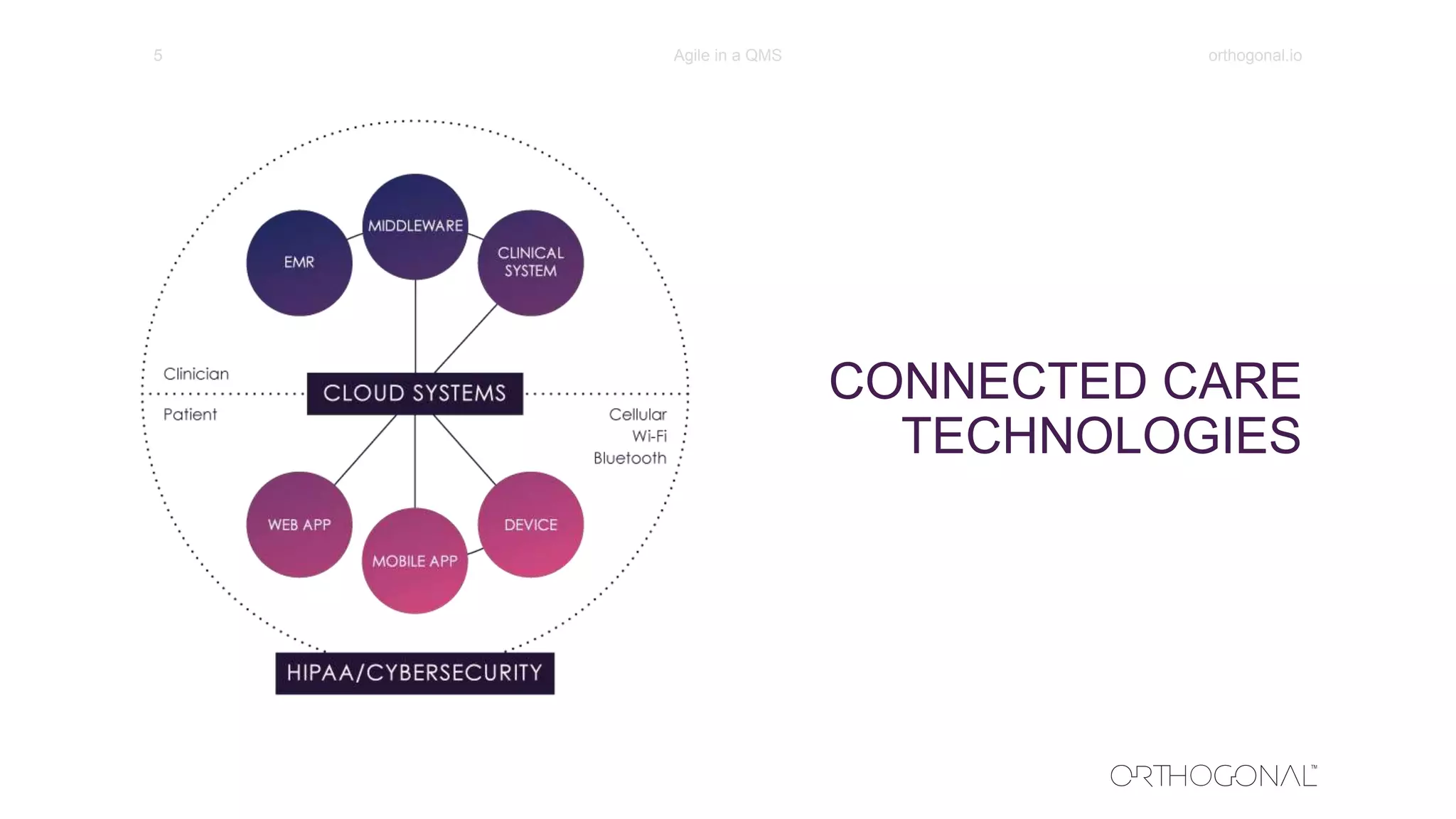
The document presents a comprehensive overview of how medical device companies can adopt agile methodologies in their Quality Management Systems (QMS). It discusses the benefits, challenges, and implementation strategies for integrating agile practices, emphasizing iterative risk management, verification and validation processes, and the importance of continuous feedback. Key factors for successful agile deployment include addressing regulatory complexities and ensuring the adaptation of agile principles in the context of medical device standards.











![BEHAVIOR DRIVEN DEVELOPMENT
(BDD)
orthogonal.ioAgile in a QMS27
“Behavior-driven development is an
“outside-in” methodology. It starts at
the outside by identifying business
outcomes, and then drills down into
the feature set that will achieve those
outcomes. Each feature is captured as
a “story”, which defines the scope of
the feature along with its acceptance
criteria.”
• —Dan North
Title (one line describing the story)
Narrative:
As a [role]
I want [feature]
So that [benefit]
Acceptance Criteria: (presented as Scenarios)
Scenario 1: Title
Given [context]
And [some more context]...
When [event]
Then [outcome]
And [another outcome]...
Scenario 2: ...](https://image.slidesharecdn.com/agiled-medical-software-161201214445/75/Agile-in-Medical-Software-Development-27-2048.jpg)