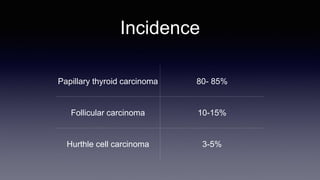
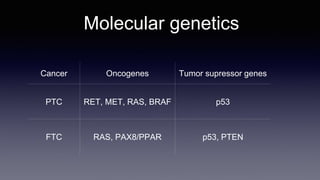
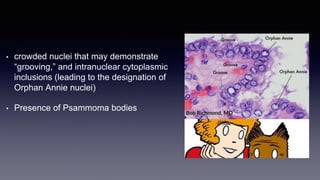
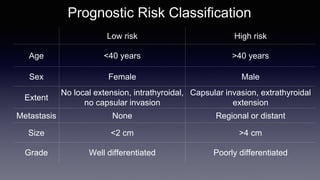
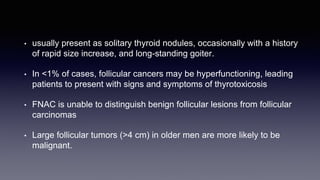
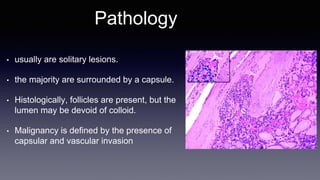
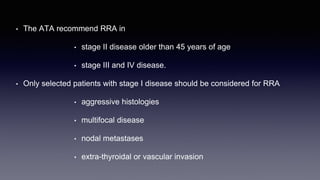
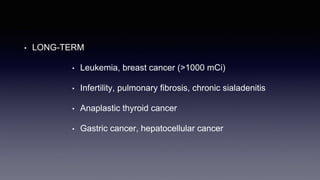
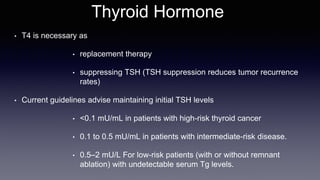
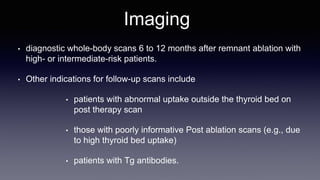
Differentiated thyroid cancer includes papillary, follicular, and Hurthle cell carcinomas which arise from thyroid follicular cells. Papillary thyroid carcinoma is the most common type, accounting for 80-85% of cases. It typically presents as a slow-growing solitary thyroid nodule and has an excellent prognosis with a 95% 10-year survival rate. Surgical treatment may include total thyroidectomy followed by radioactive iodine (RAI) therapy in high-risk cases. Long-term monitoring involves measuring serum thyroglobulin and ultrasound imaging of the neck. Localized recurrence is typically treated with surgery while metastatic disease is managed with additional RAI if radioiodine avid or other modalities if not