Lecture 11 recombinant protein production
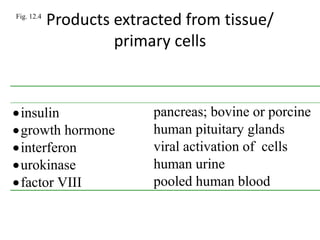
- 1. Fig. 12.4 Products extracted from tissue/ primary cells Product Extracted from.... insulin pancreas; bovine or porcine growth hormone human pituitary glands interferon viral activation of cells urokinase human urine factor VIII pooled human blood
- 2. Fig. 12.5 Problems of extraction from animal/ human sources • small quantities available • non-human proteins cause immunogenicity • contamination with viruses or prions - Creutzfeld-Jakob disease - HIV from blood
- 3. .17
- 4. Lecture 14 - Animal Cell Biotechnology Animal cell products – Recombinant proteins 1. Insulin • hormone produced by beta cells in the pancreas → allows glucose to pass into cells → suppresses excess production of sugar in the liver and muscles → suppresses breakdown of fat for energy
- 8. Lecture 14 - Animal Cell Biotechnology Animal cell products – Recombinant proteins beta cells in pancreas preproinsulin proinsulin insulin + C-peptide Butler, M. 1987. Animal cell technology: principles and products. Stony Stratford: Open University Press. P107.
- 9. Computer-generated image of insulin hexamers highlighting the threefold symmetry, the zinc ion holdin it together and the histidine residues invlolved in zinc- binding Iinsulin 51 amino acids 5,8808 molecular weight
- 10. Lecture 14 - Animal Cell Biotechnology Animal cell products – Recombinant proteins • insulin produced from pig pancreas cells → structure of insulin differs slightly between species → the C-terminal amino acid of the B chain = alanine (threonine in humans) • two problems associated with porcine insulin → causes immunogenic response in some diabetic patients → supply of pancreas fluctuates with meat trade
- 11. Fig. 12.6 Pig to human insulin A (21) S- S S S Thr B (30) S S B 30 Ala
- 12. Producing A and B chains separately
- 13. Lecture 14 - Animal Cell Biotechnology Animal cell products – Recombinant glycoproteins 2. Interferons • glycoproteins that “interfere” with viral propagation in cell cultures • group of small proteins with 140-170 amino acids • secretory protein produced from viral-infected cells, induces antiviral state in neighboring cells
- 14. Interferon interferes with viral replication in protected cells Butler, M. 1987. Animal cell technology: principles and products. Stony Stratford: Open University Press. P70.
- 15. Lecture 14 - Animal Cell Biotechnology Animal cell products – Recombinant glycoproteins 3 main types of interferons: 1. IFN-α (25 subtypes) – produced from β -lymphocytes 2. IFN-β – fibroblasts – produced from fibroblasts 3. IFN-γ – T-lymphocytes – produced from T-lymphocytes • mode of action not fully understood → synthesis of host enzymes that degrade viral RNA and inhibit protein synthesis
- 16. Lecture 14 - Animal Cell Biotechnology Animal cell products – Recombinant glycoproteins 5. Erythropoietin (EPO) • glycoprotein hormone produced by the kidney (hypoxia triggers EPO production) • required for continuous red blood cell production in bone marrow (erythropoiesis) • absence of EPO results in impairment of red blood cell production → anemia • anemia treated with exogenous EPO
- 17. Physiological role of erythropoietin • Hematopoietic growth factor • Produced in the kidney • Stimulates red blood cell (erythrocyte) maturation • Induces homodimerization of 2 receptor molecules • Initiates intracellular signalling cascade
- 18. Therapeutic uses of EPO Treatment of anaemia caused by :- • chronic renal failure • partial renal failure • AIDS • cancer chemotherapy • autologous transfucion
- 19. Molecular characteristics of EPO • Molecular weight: 39 kDa • 165 amino acids • Carbohydrate component: 35-40% • 3 N-linked glycans to Asn at positions 24, 38, 83 • 1 O-linked glycan to Ser at position 126
- 20. Fig. 12.11 Structure of erythropoietin
- 21. Predicted structure of glycosylated human erythropoietin The predicted structure of glycosylated protein human Erythropoietin . N- and O-glycans were added to the core protein structure (pdbid 1BUY) using the Glycoprotein Builder tool at the GLYCAM-Web site (www.glycam.com). High mannose N-linked glycans (Man9GlcNAc2) were added at ASN 24, 38 and 83 and one O-linked glycan (a-GalNAc) at Ser126. (R.Woods)
- 22. Fig. 12.12 Recombinant human Erythropoietin Non-glycosylated Glycosylated Asn83 Asn38 Asn24 Ser126 39 kDa 18 kDa
- 23. Tetra-antennary N-glycan structure 1-4 Asn-X-Ser/Thr 1-6 1-6 = Fuc Asn = GlcNAc 1-3 8 = Man 6 1-2 4 = Gal 3 2 = NeuAc 2-3 Linkage position Complex -linkage -linkage tetra-antennary
- 24. Lecture 14 - Animal Cell Biotechnology Animal cell products – Recombinant glycoproteins carbohydrates make up ~40% (by weight) of glycoprotein → important for full activity in vivo allows EPO to remain in circulation (removed by liver) Egrie and Browne (2001) developed a novel form of EPO (novel erythropoiesis-stimulating protein (NESP)) hyper-glycosylated form of EPO with greater half-life (3x half life of EPO)
- 25. Fig. 12.13 Variant glycoforms of recombinant Epo and NESP Maximum number of sialic acid groups in glycoform 22 (NESP) 14 12 8 O-linked glycan N-linked glycans
- 26. Fig. 12.15 The biological activity of each isoform of Epo after a 30-day treatment Increase in hematocrit from baseline 30 25 20 15 10 5 0 8 9 10 11 12 13 14 Number of sialic acid groups in Epo isoform
- 27. Fig. 12.14 Serum half-life of analogues of Epo with variable N-glycan sites 7 6 5 serum half-life (h) 4 3 2 1 0 rEpo 4-glycan NESP Epo type
- 28. Lecture 14 - Animal Cell Biotechnology Animal cell products – Recombinant glycoproteins 3. Plasminogen activators • thrombosis (formation of blood clots) is a major cause of premature death • deposition of fibrin in the circulatory system, blocks blood flow • formation of insoluble fibrin controlled by clotting cascade formed during wound healing • t-PA (tissue-plasminogen activator) initiates fibrinolysis (proteolytic cleavage of fibrin)
- 29. Therapeutic applications • t-PA is used in diseases that feature blood clots, - - pulmonary embolism - myocardial infarction - stroke to be effective, t-PA must be administered within the first 3 hours/ to be given intravenously,
- 30. Fig. 12.9 disulphide bond N-glycan
- 31. Lecture 14 - Animal Cell Biotechnology Animal cell products – Recombinant glycoproteins Fibrinolysis Tissue-plasminogen activator Plasminogen Plasmin Coagulation Fibrin Fibrin products (insoluble) (soluble)
- 32. Lecture 14 - Animal Cell Biotechnology Animal cell products – Recombinant glycoproteins • gene for t-PA transfected into CHO-K1 cells, one of the first recombinant products derived from mammalian cells in 1987 → secreted in vivo by a number of tissues → production stimulated by a number of substances, including thrombin and histamine → half-life of t-PA varies from 2-4 min
- 33. Lecture 14 - Animal Cell Biotechnology Animal cell products – Recombinant glycoproteins 4. Blood-clotting factors • Hemophilia is a sex-linked (x-chromosome) genetic disease • inactive clotting cascade in blood, can’t form fibrin → hemophilia A – absence of factor VIII → hemophilia B – absence of factor IX
- 34. The clotting cascade Wound surface contact Factor XII Factor XIIa Factor XI Factor XIa Factor IX Factor IXa +Factor VIII + Thrombin Factor X Factor Xa +Factor V Prothrombin Thrombin Fibrinogen Fibrin clot
- 35. Fig. 12.10 The clotting cascade Wound surface contact Factor XII Factor XIIa Factor XI Factor XIa Factor IX Factor IXa + Factor VIII + Thrombin Factor X Factor Xa +Factor V Prothrombin Thrombin Fibrinogen Fibrin clot
- 36. Lecture 14 - Animal Cell Biotechnology Animal cell products – Recombinant glycoproteins Factor VIII • large glycoprotein (265 kDa) • gene – 186 kB, 26 exons, 25 introns (overlapping strands of DNA from genomic and cDNA aligned, without introns) • BHK cells transfected with expression vector containing gene encoding Factor VIII • produces biologically active protein with correct tertiary folding and glycosylation • stabilized by addition of Willebrand factor, normally found as a combined protein complex in blood
- 37. Lecture 14 - Animal Cell Biotechnology Animal cell products – Recombinant glycoproteins Factor IX • plasma glycoprotein (57 kDa) secreted by hepatocytes • called “Christmas factor”, after first family diagnosed with clotting deficiency • gene cloned into rat hepatoma cell line → contains enzymes for post-translation modifications
- 39. Lecture 14 - Animal Cell Biotechnology Animal cell products – Artificial skin • important for skin grafting (i.e. for severe burn victims) • one method described by Hardin-Young and Parenteau 2002) → dermal-equivalent formed from fibroblasts → epidermal equivalent formed from keratinocytes • keratinocytes and fibroblasts are derived from neonatal foreskin tissue, lack antigen presentation
- 40. Fig. 12.16 The principle of gene therapy ex vivo