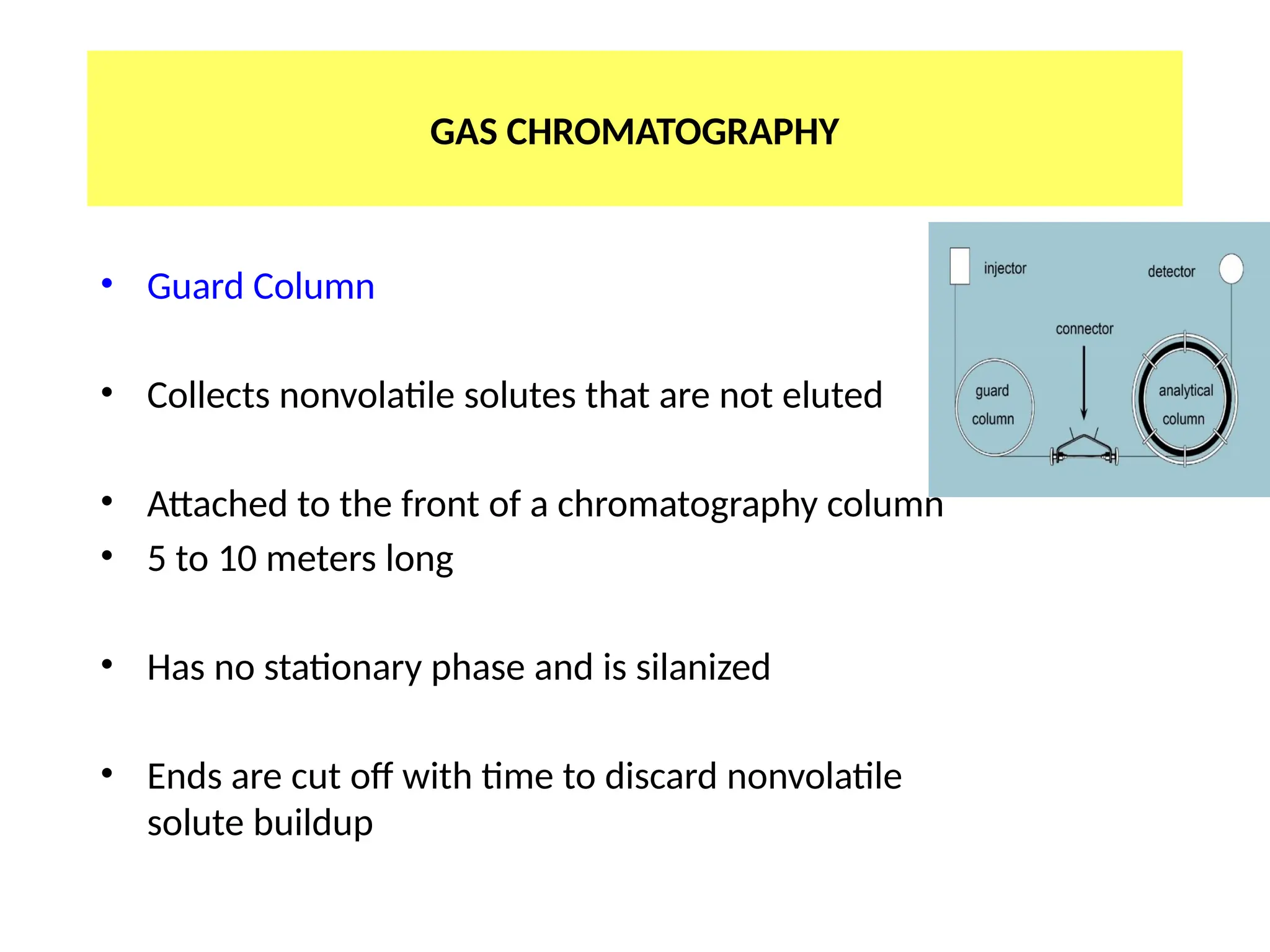
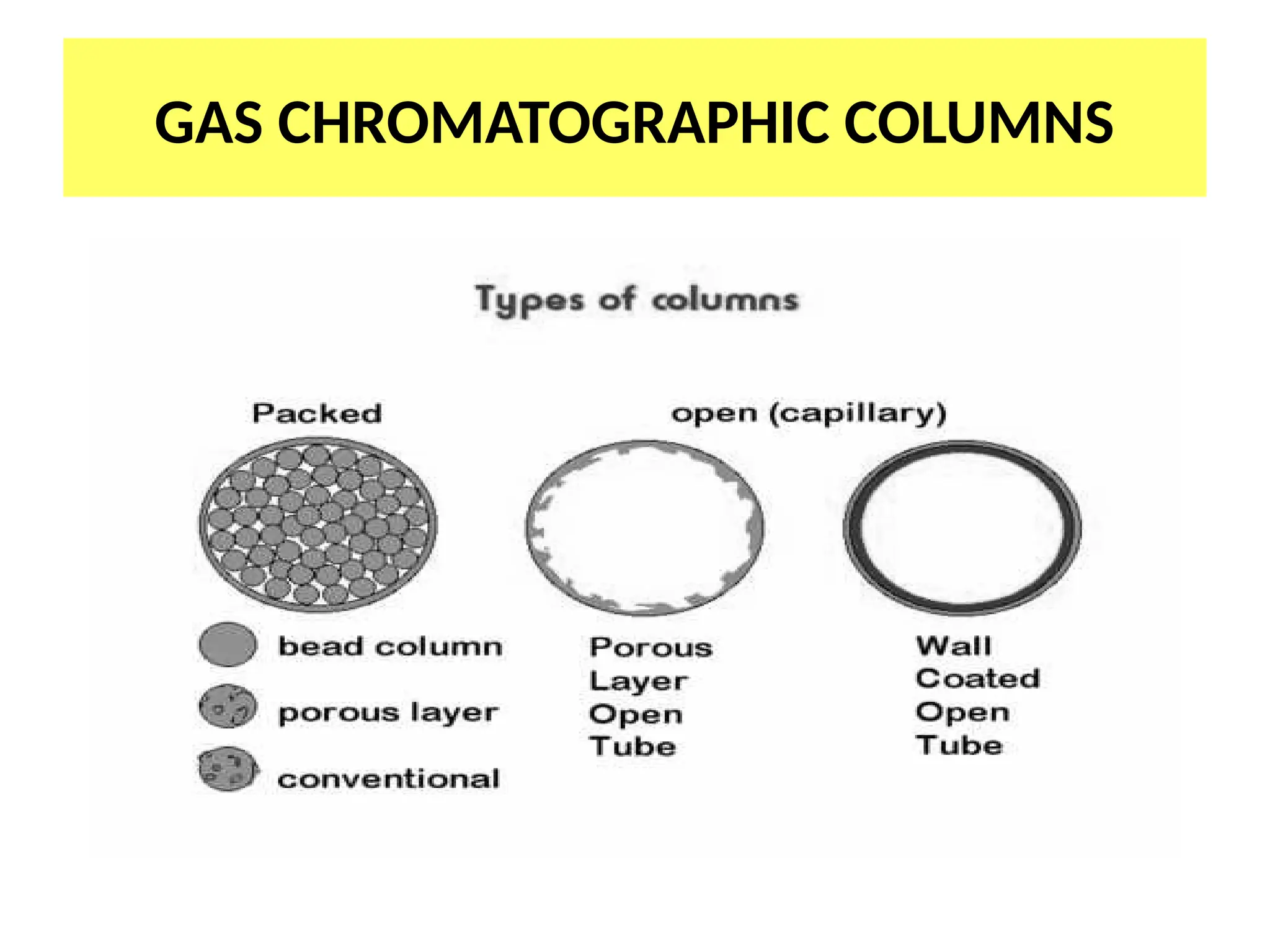
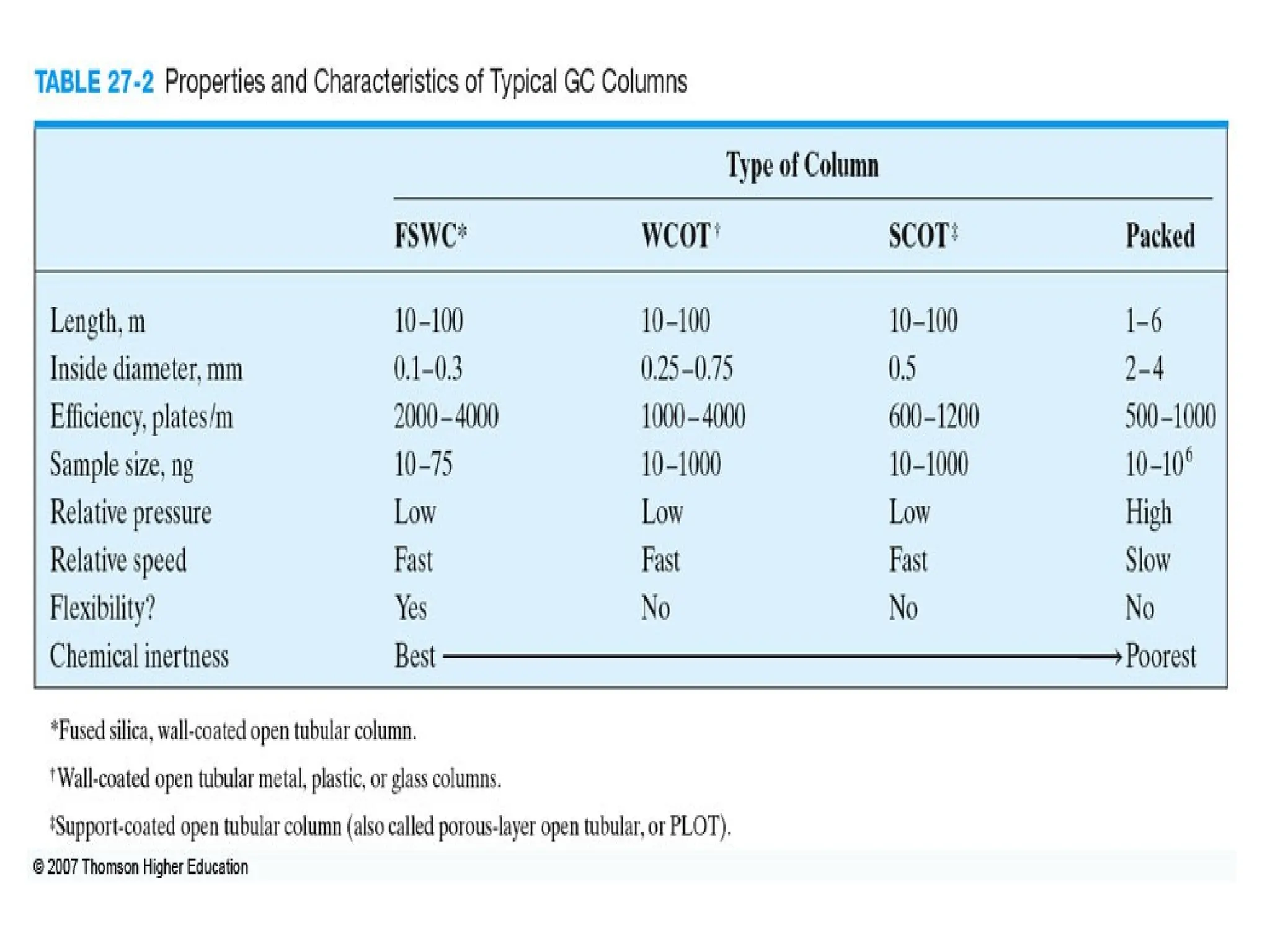
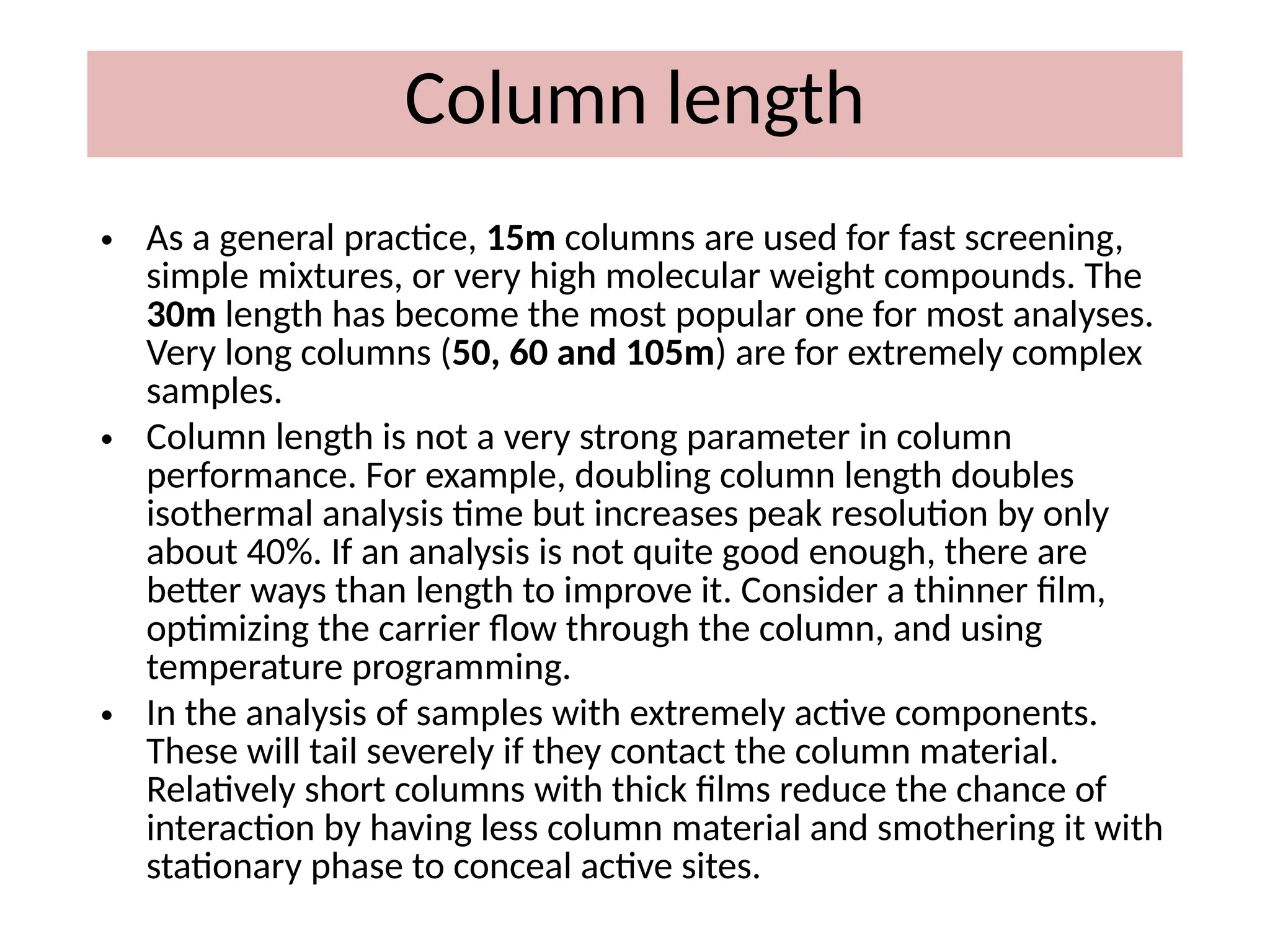
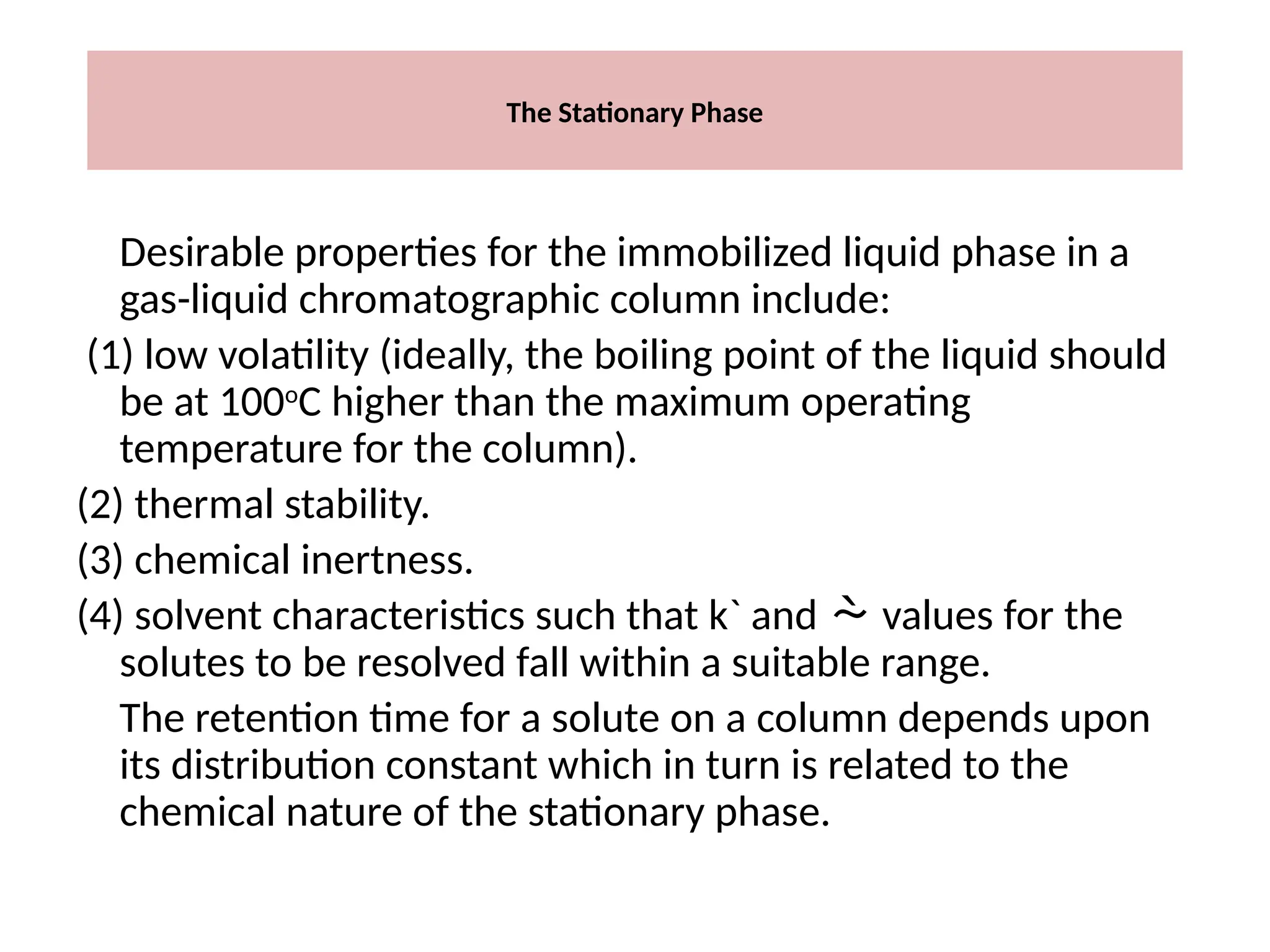
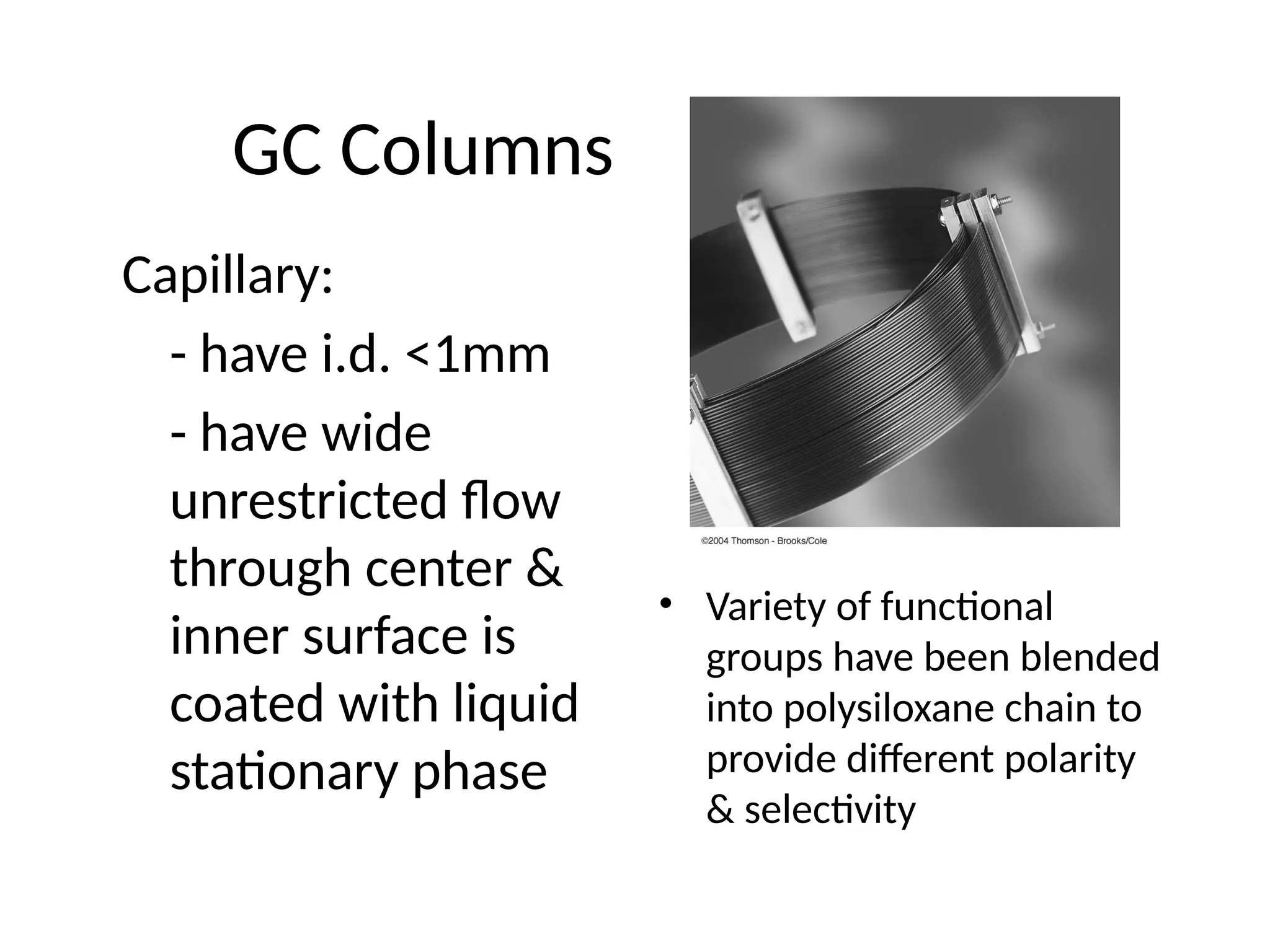
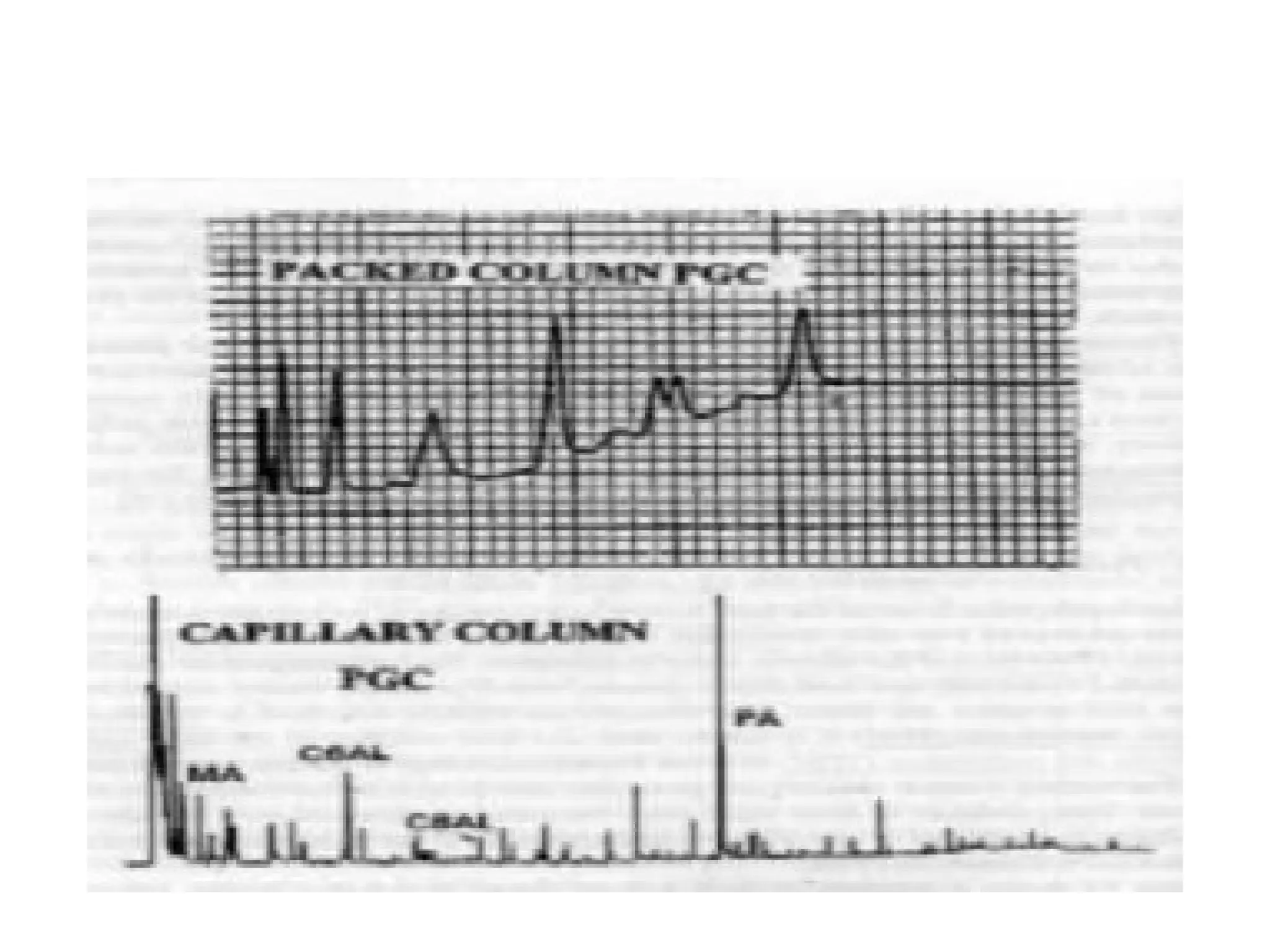
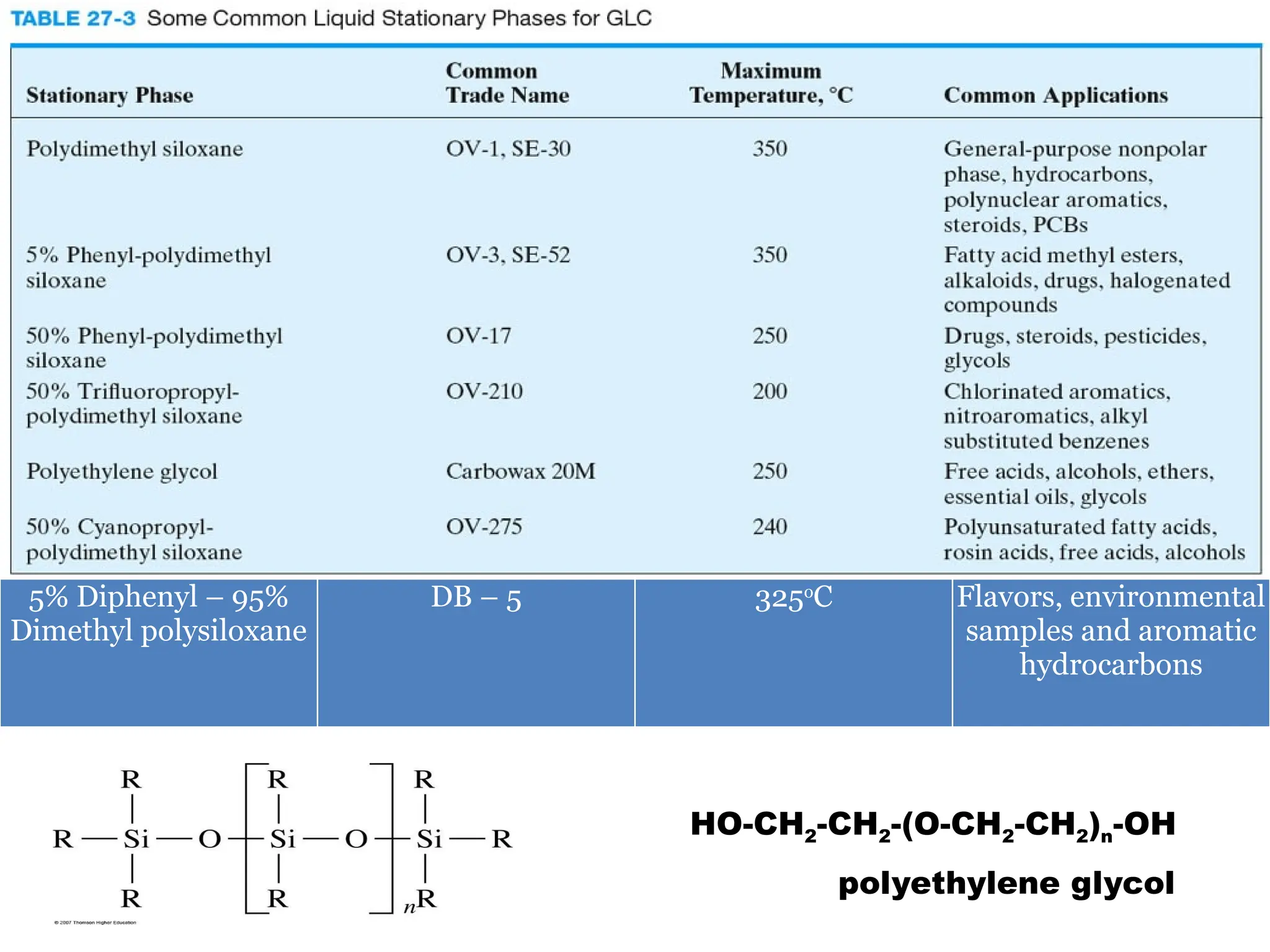
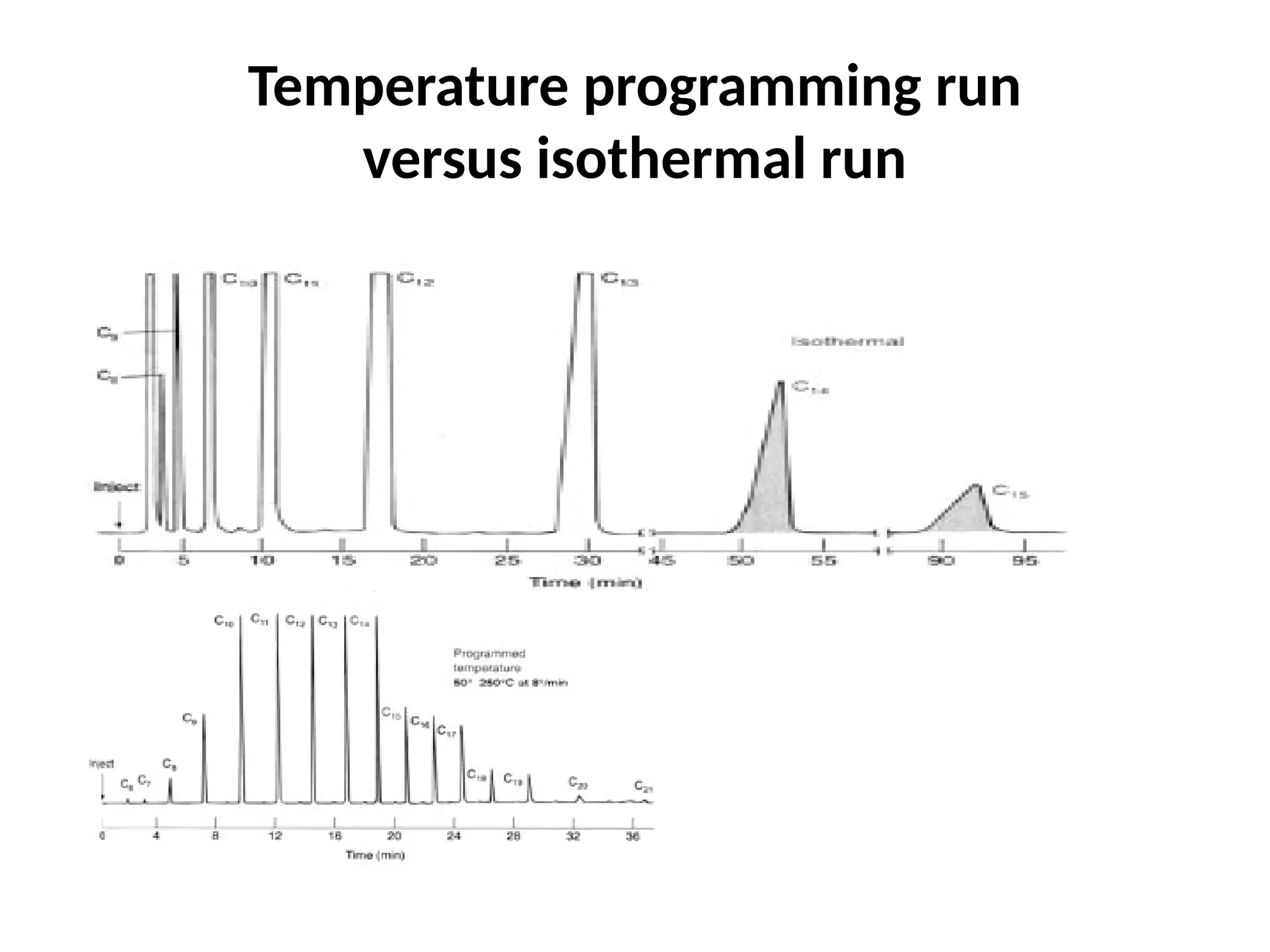
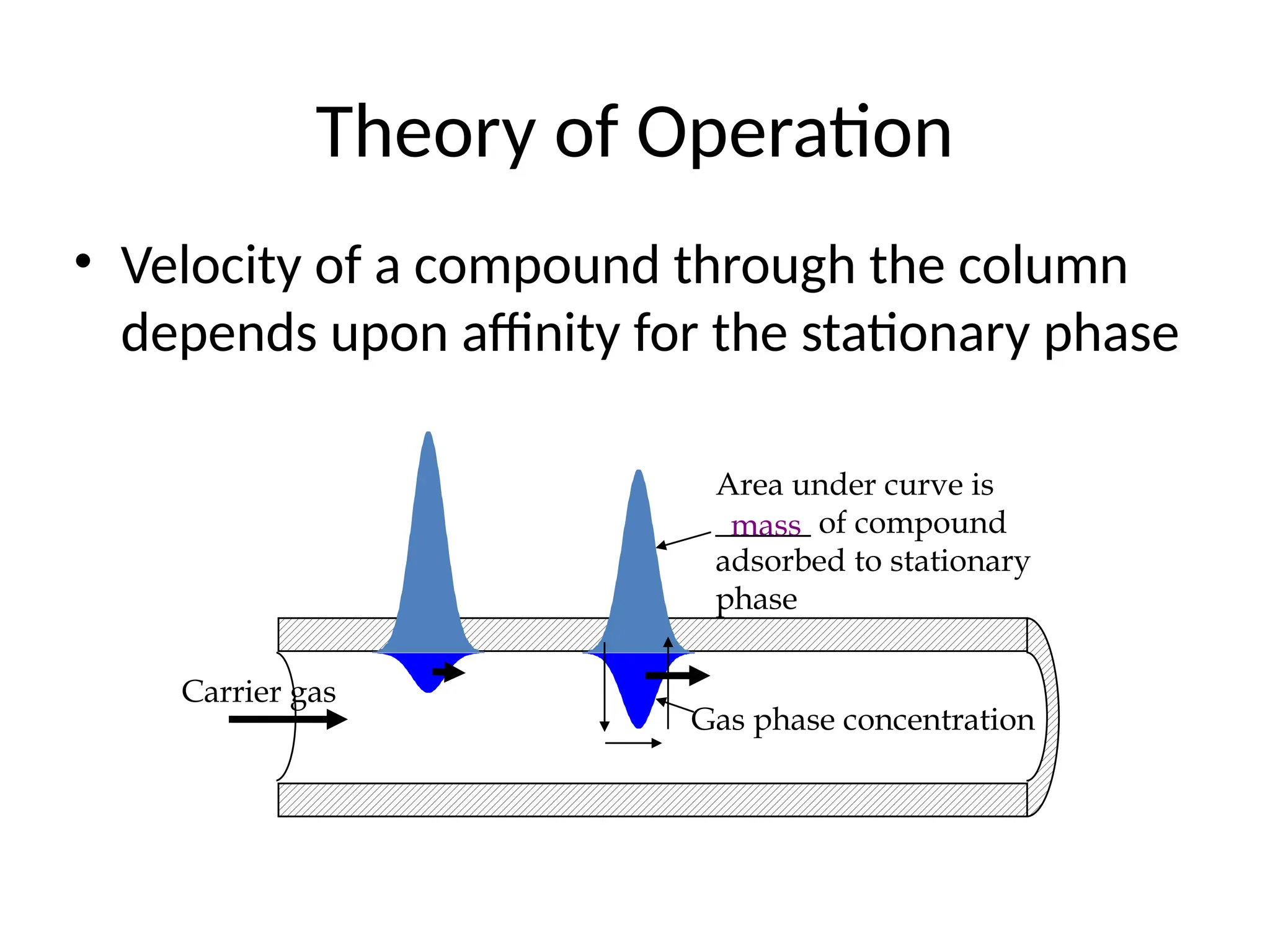
The document discusses gas chromatography columns, detailing types such as packed and open tubular (capillary) columns, their construction materials, and common lengths used for various analyses. It highlights the importance of temperature control in the chromatography process, including the use of temperature programming to optimize separation. Additionally, it addresses the effects of column length and stationary phase properties on performance and resolution in analyses.