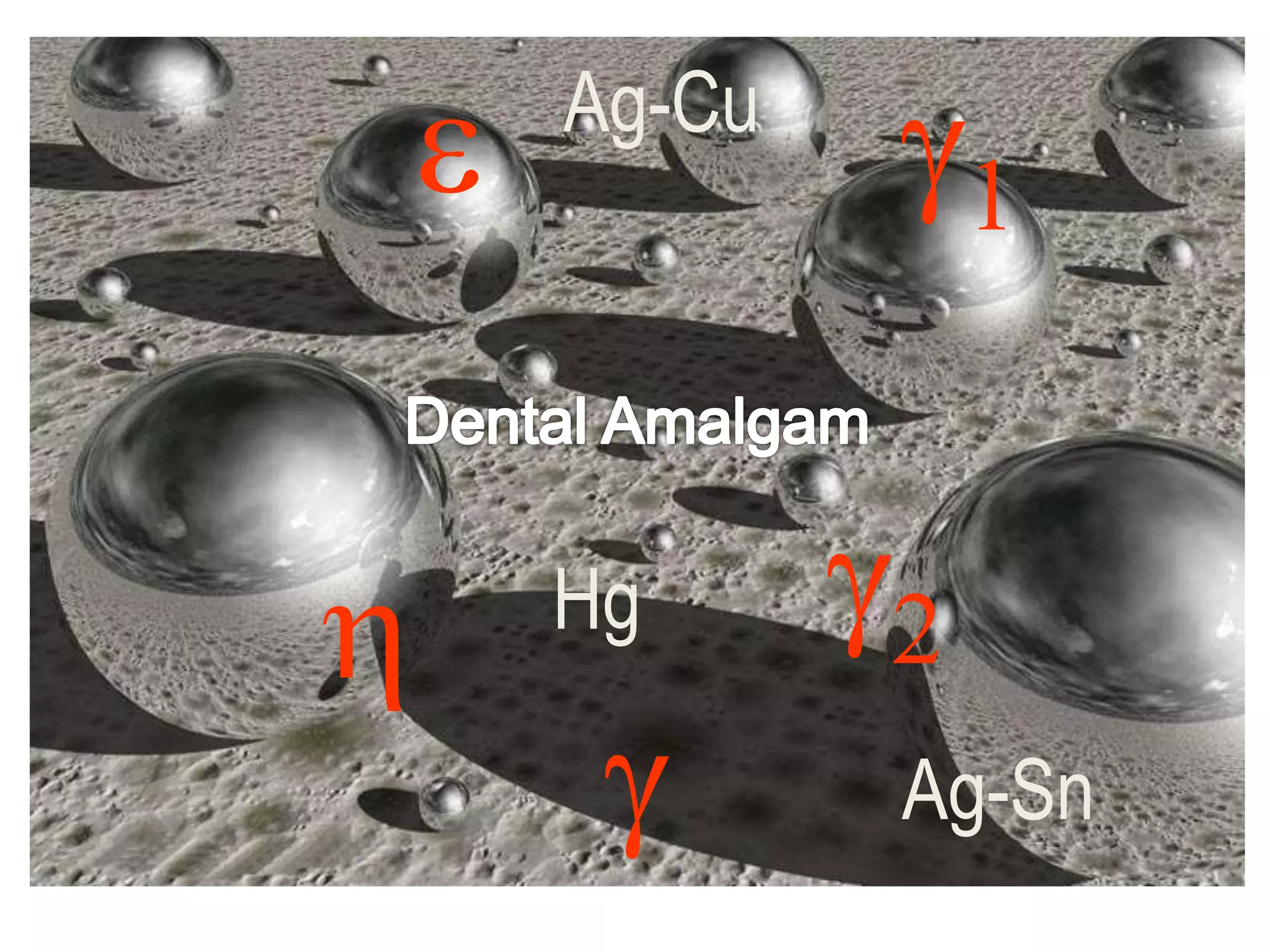
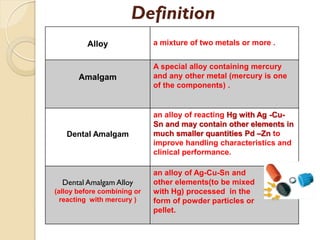
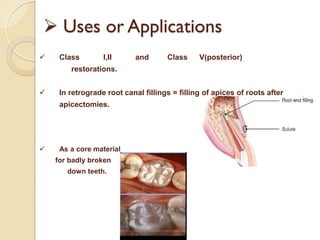
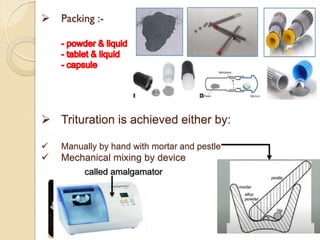
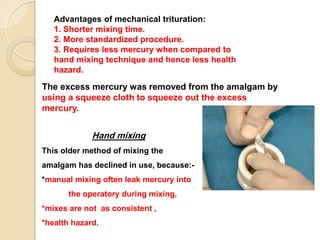
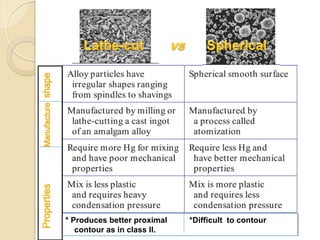
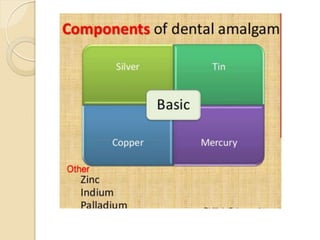
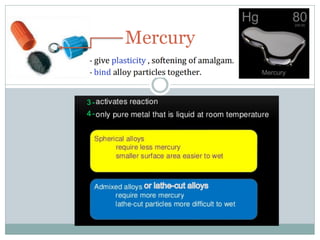
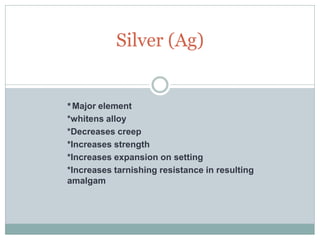
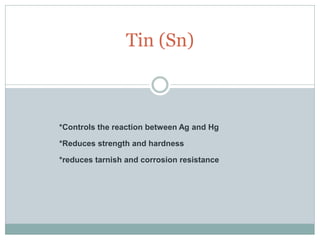
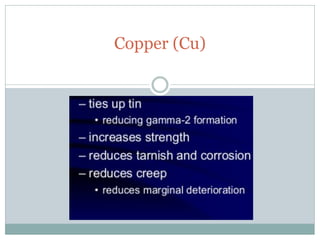
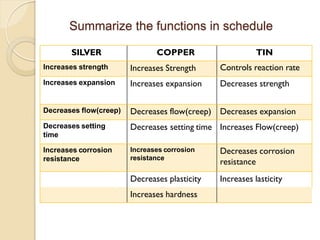
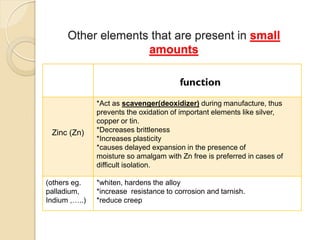
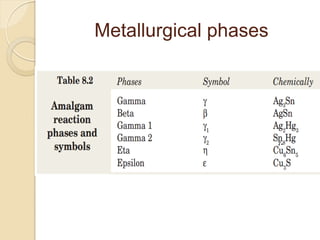
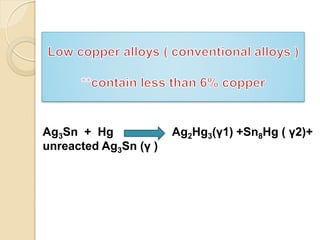
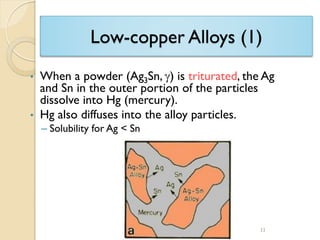
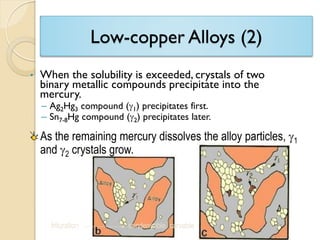
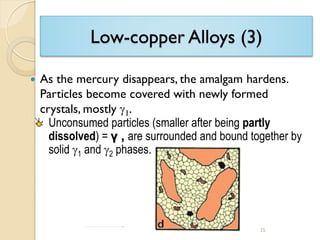
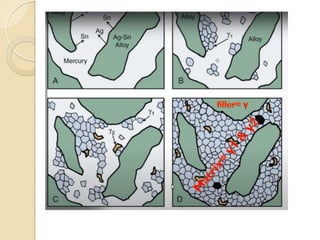
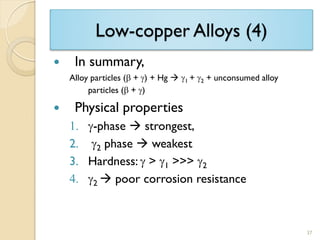
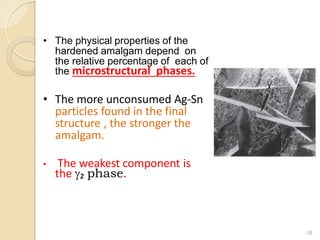
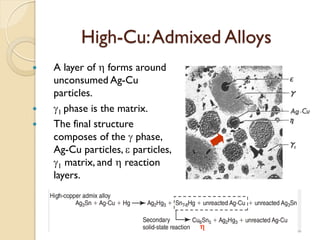
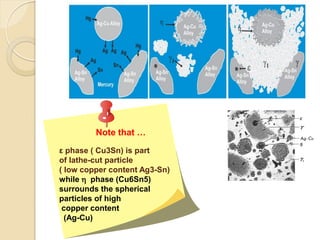
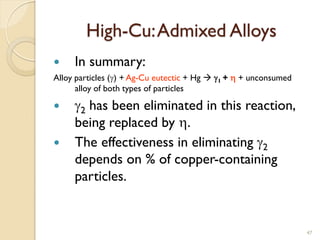
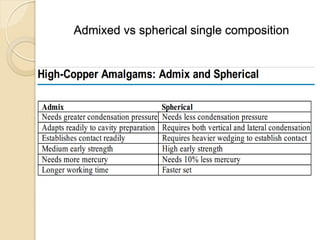
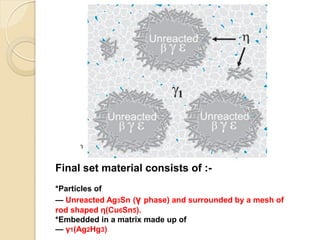
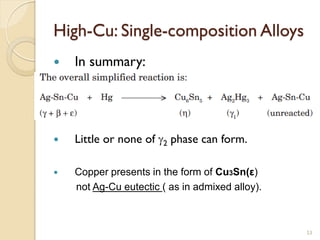
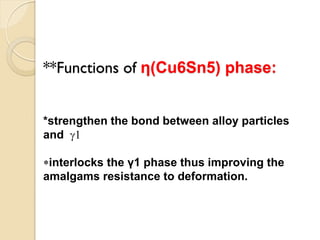
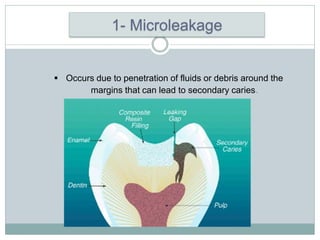
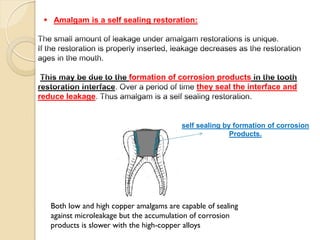
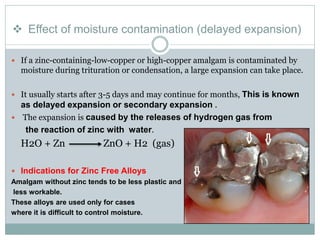
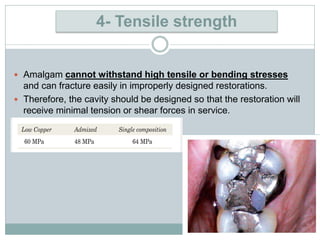
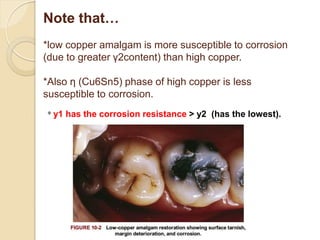
Dental amalgam, an alloy containing mercury, silver, and copper, has been used for over a century as a durable filling material for tooth restoration. The document details the properties, classifications, and functions of dental amalgams, emphasizing the differences between low and high copper alloys, particularly their mechanical properties and clinical performance. Additionally, it discusses the process of amalgam preparation, trituration methods, and the impact of environmental factors on amalgam integrity.