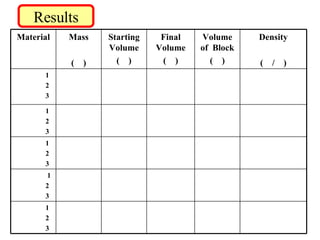
This document provides instructions for calculating the density of different metal blocks through measuring their mass and volume. Students are directed to find the mass of each block using a balance and then measure its volume by finding the displacement of water in a graduated cylinder. They then calculate the density of each block by dividing its mass by volume. The procedure is repeated multiple times for accuracy and repeated for additional blocks to compare densities.