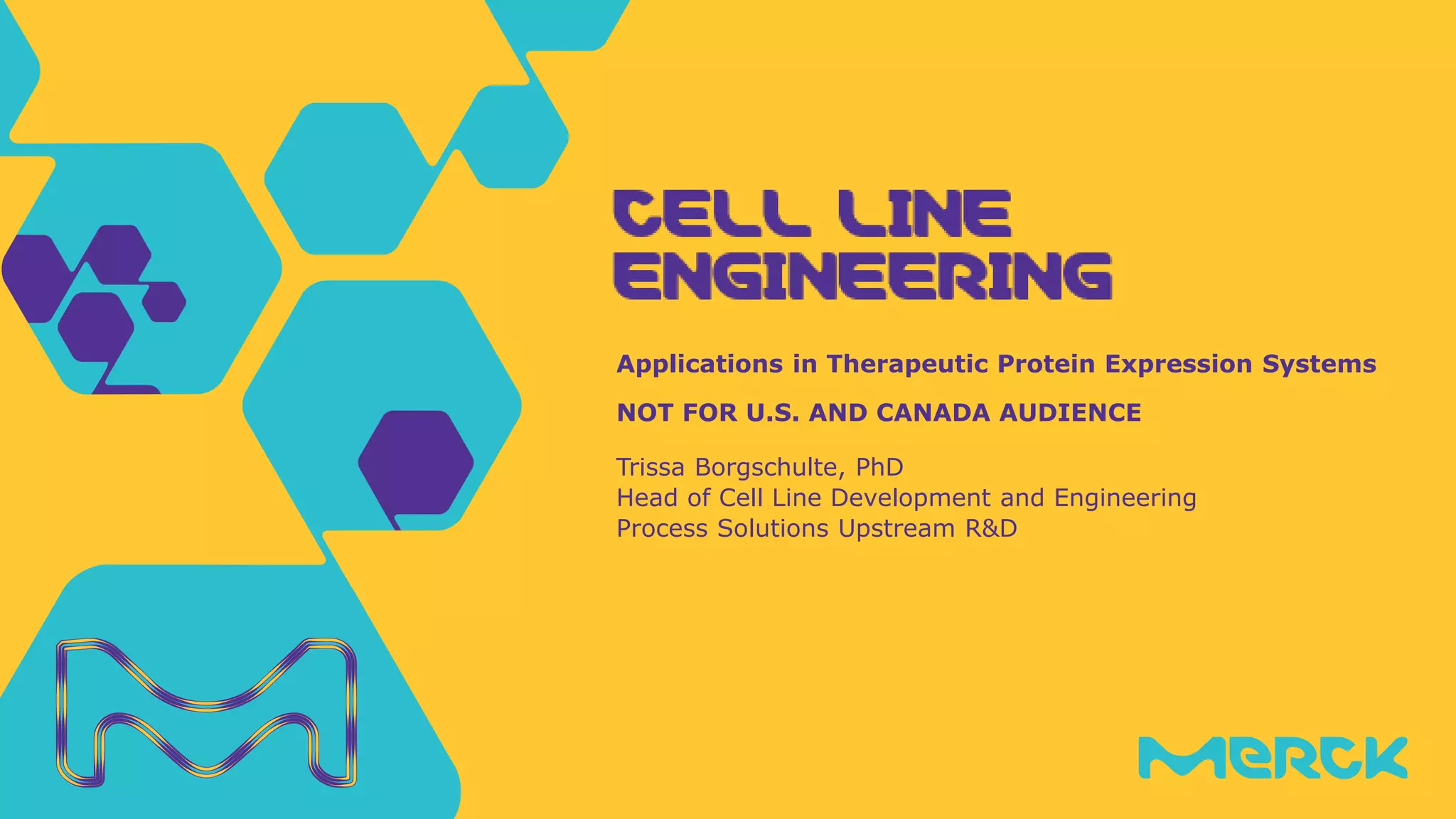
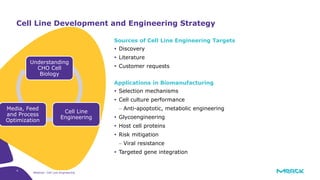
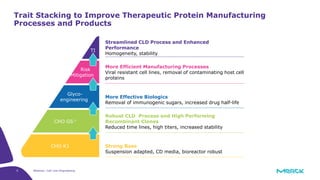
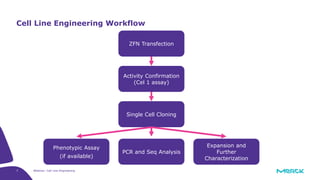
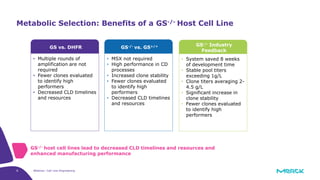
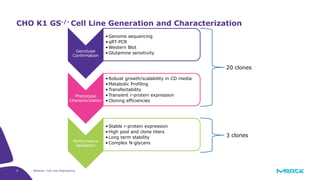
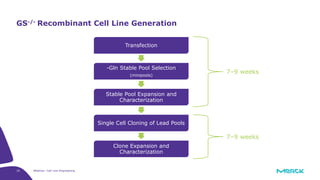
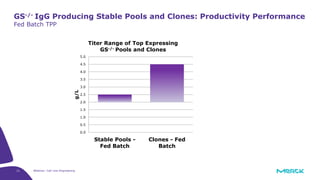
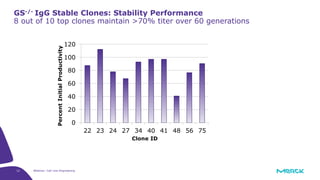
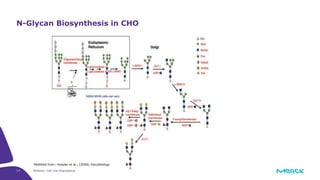
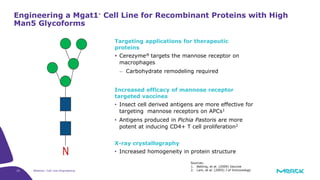
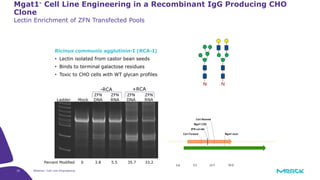
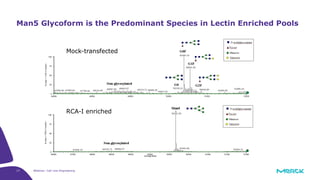
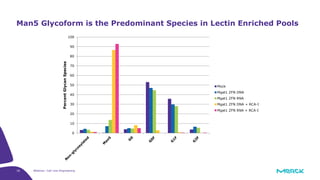
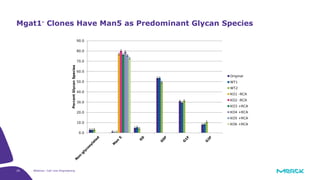
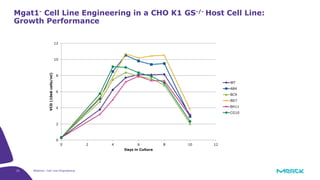
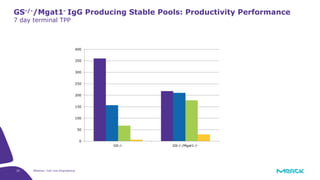
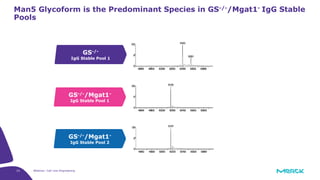
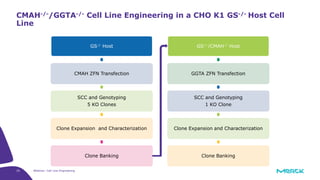
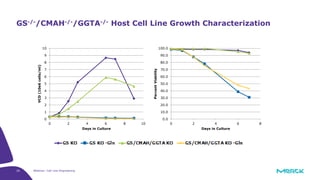
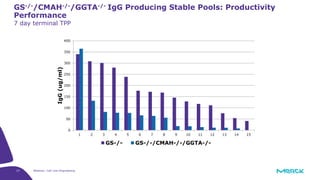
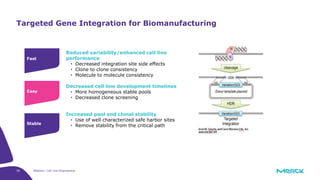
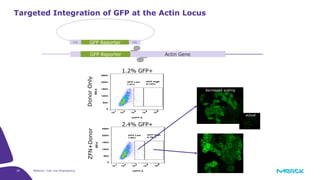
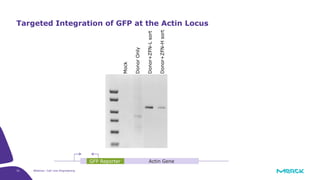
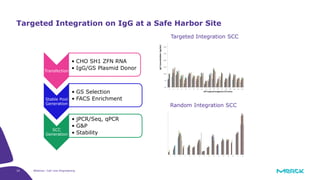
The document discusses advancements in cell line engineering, particularly using Zinc Finger Nucleases (ZFNs) for genome editing to improve therapeutic protein manufacturing efficiency and clone stability. It highlights the benefits of using GS-/- host cell lines and glycoengineering in biomanufacturing processes to enhance product quality and regulatory compliance. Additionally, it addresses targeted gene integration for consistent and robust cell line performance, ultimately aiming to streamline production timelines and increase therapeutic effectiveness.