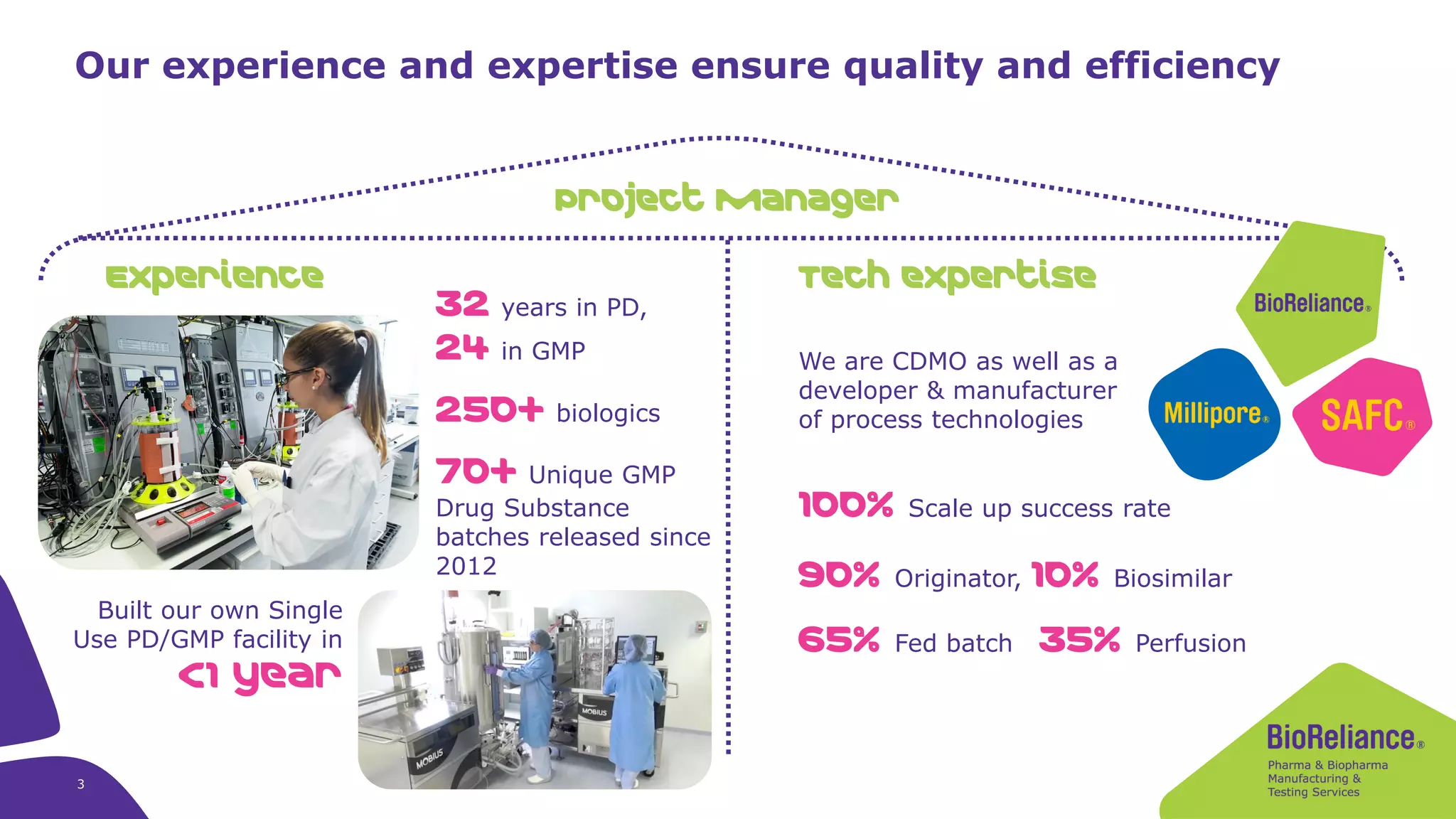
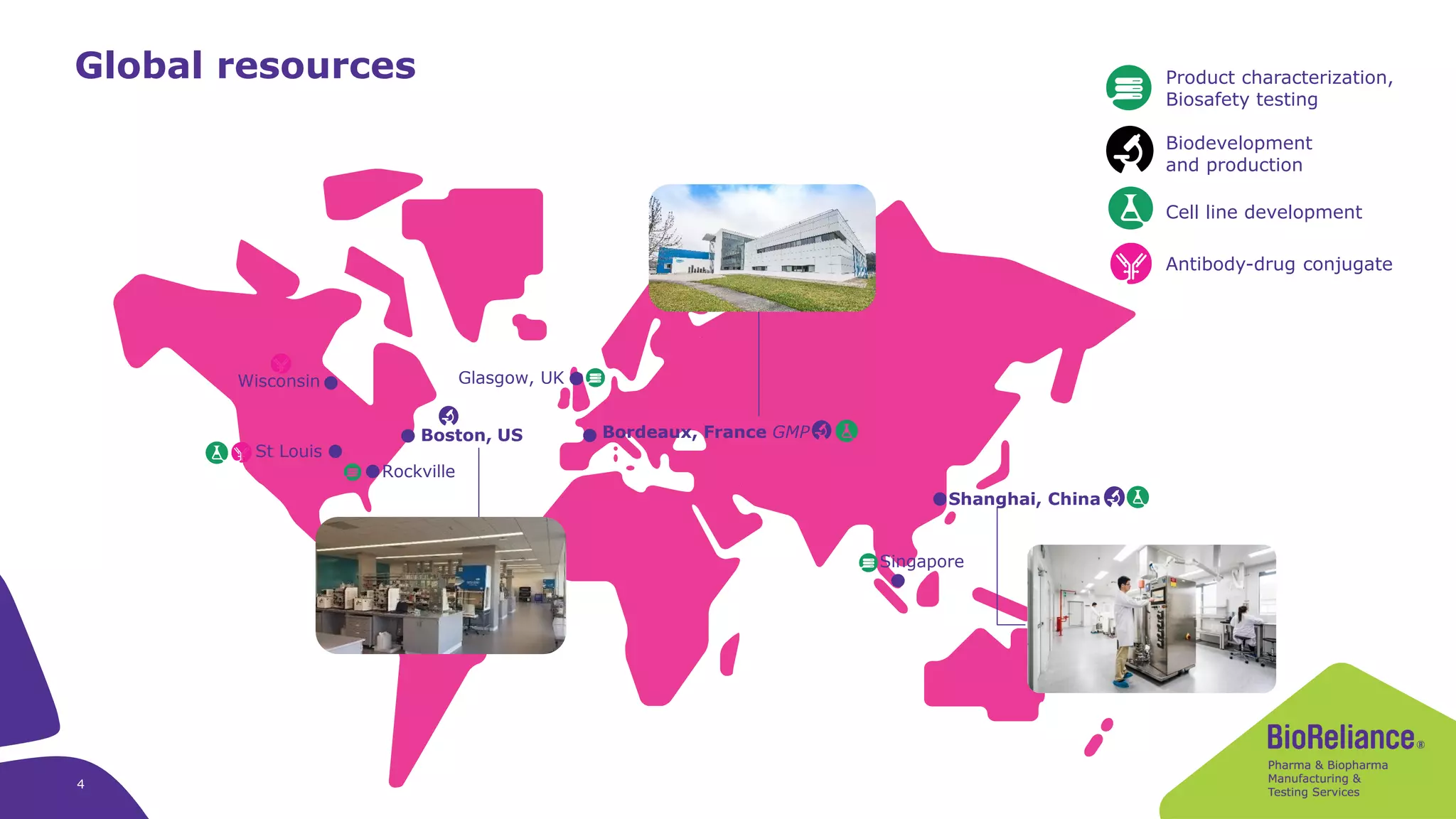
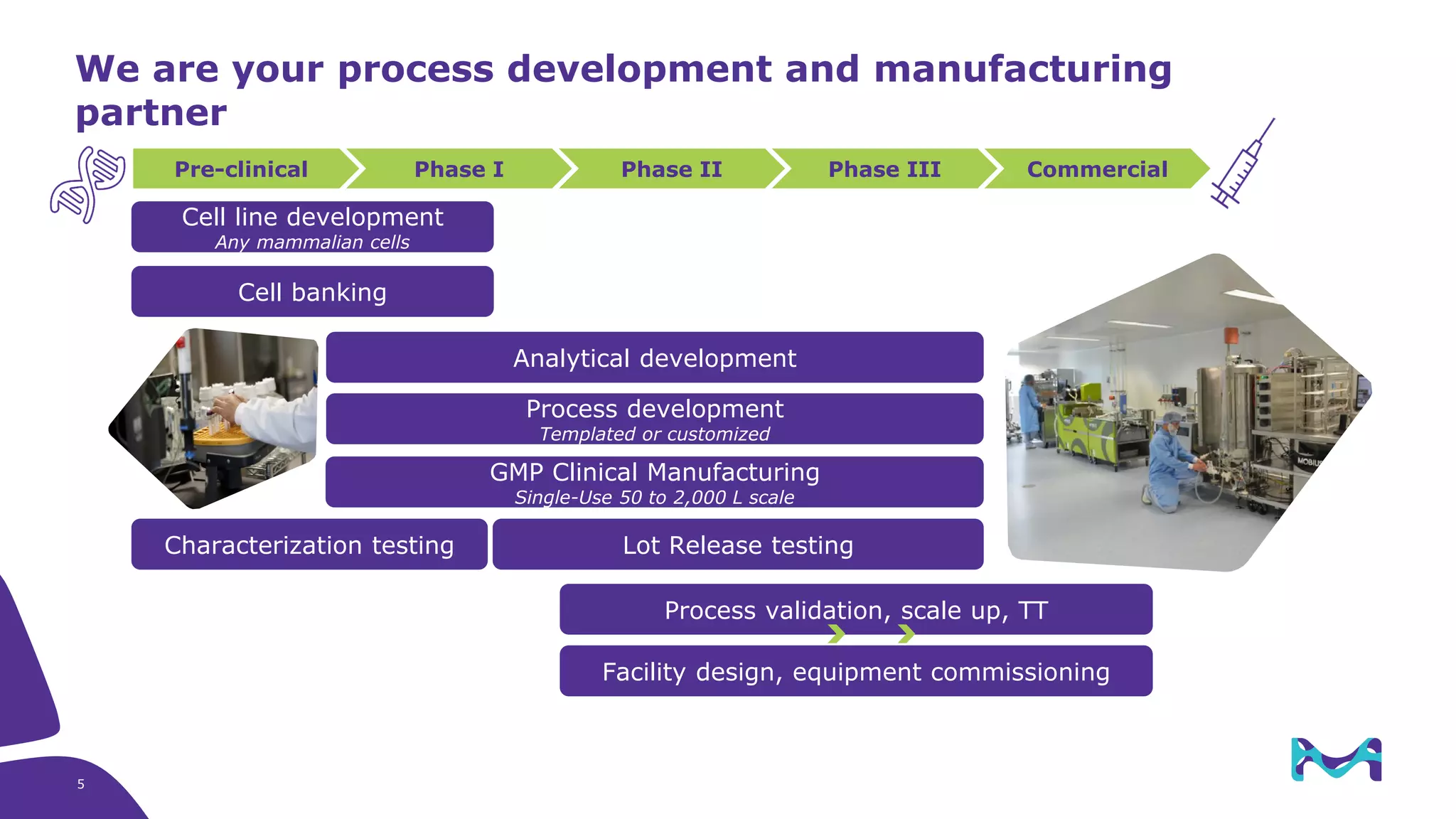
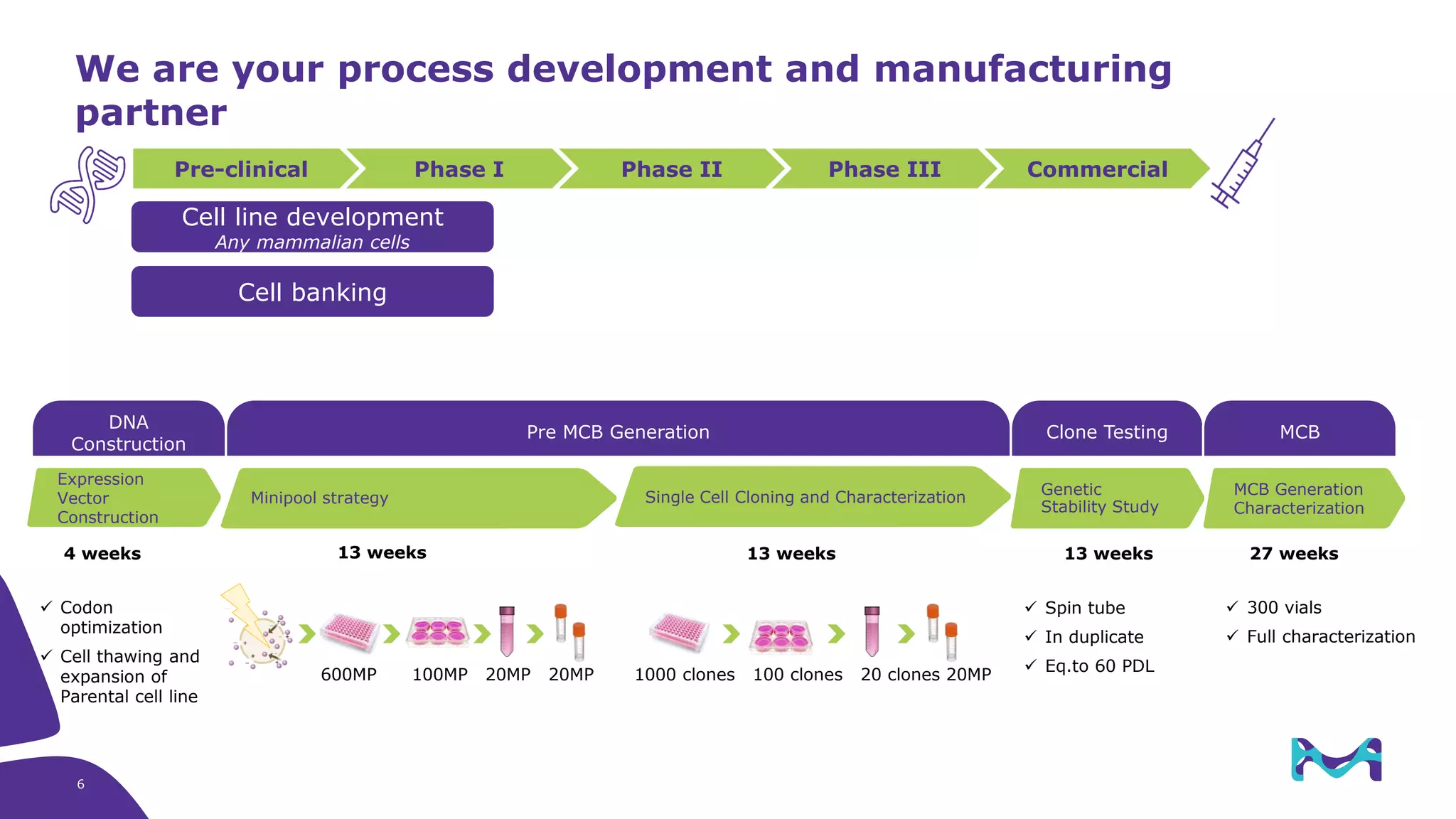
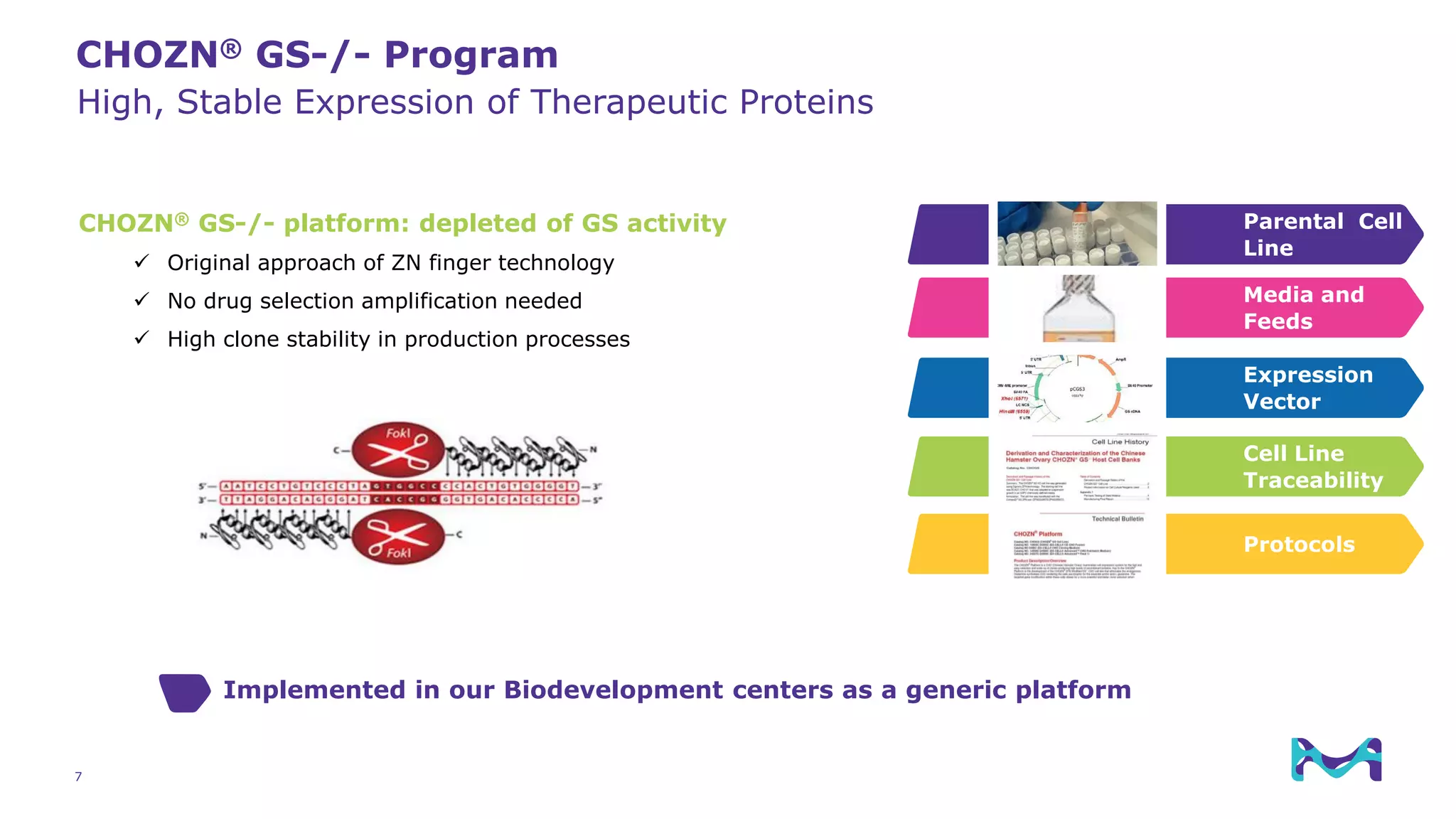
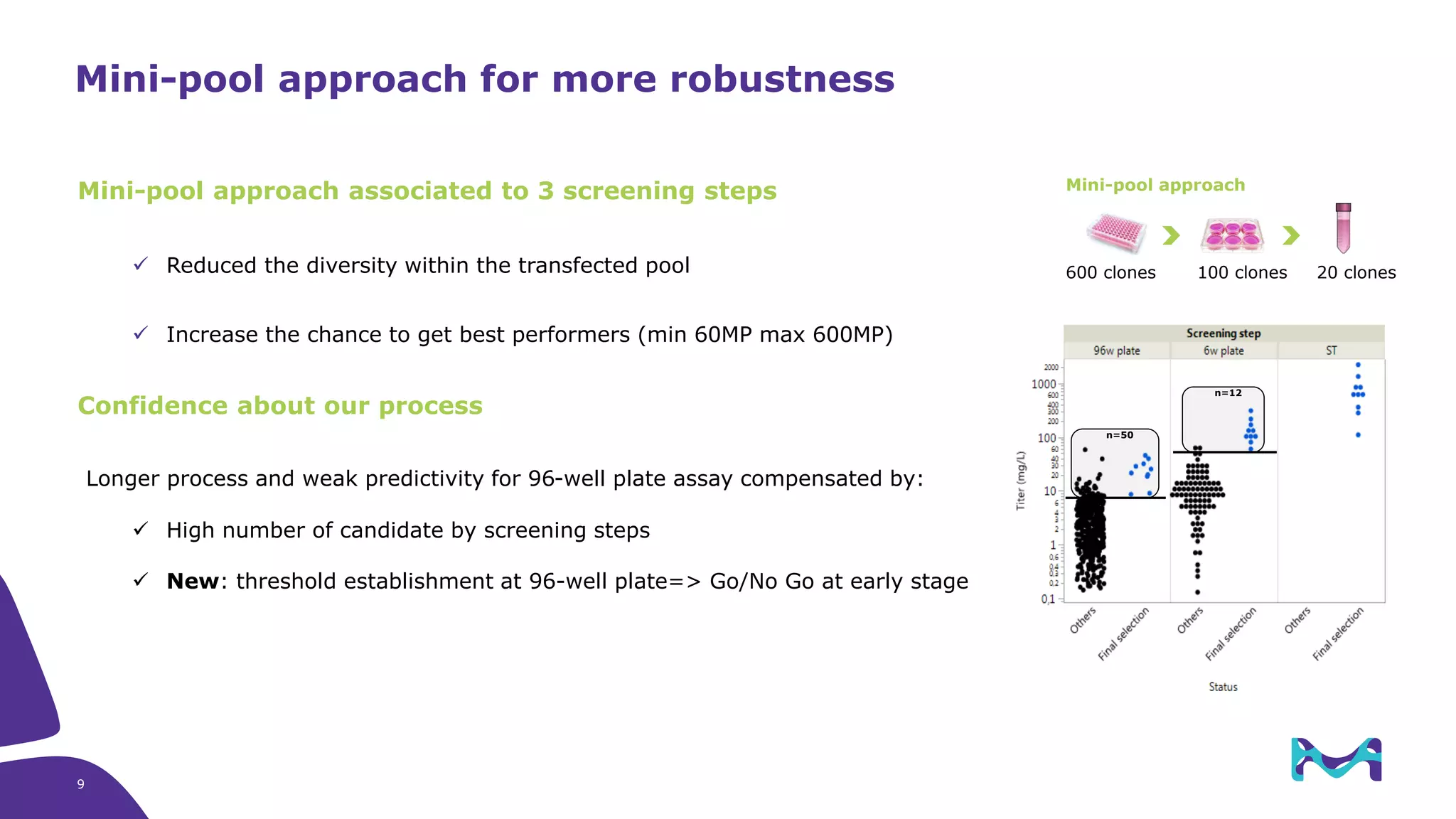
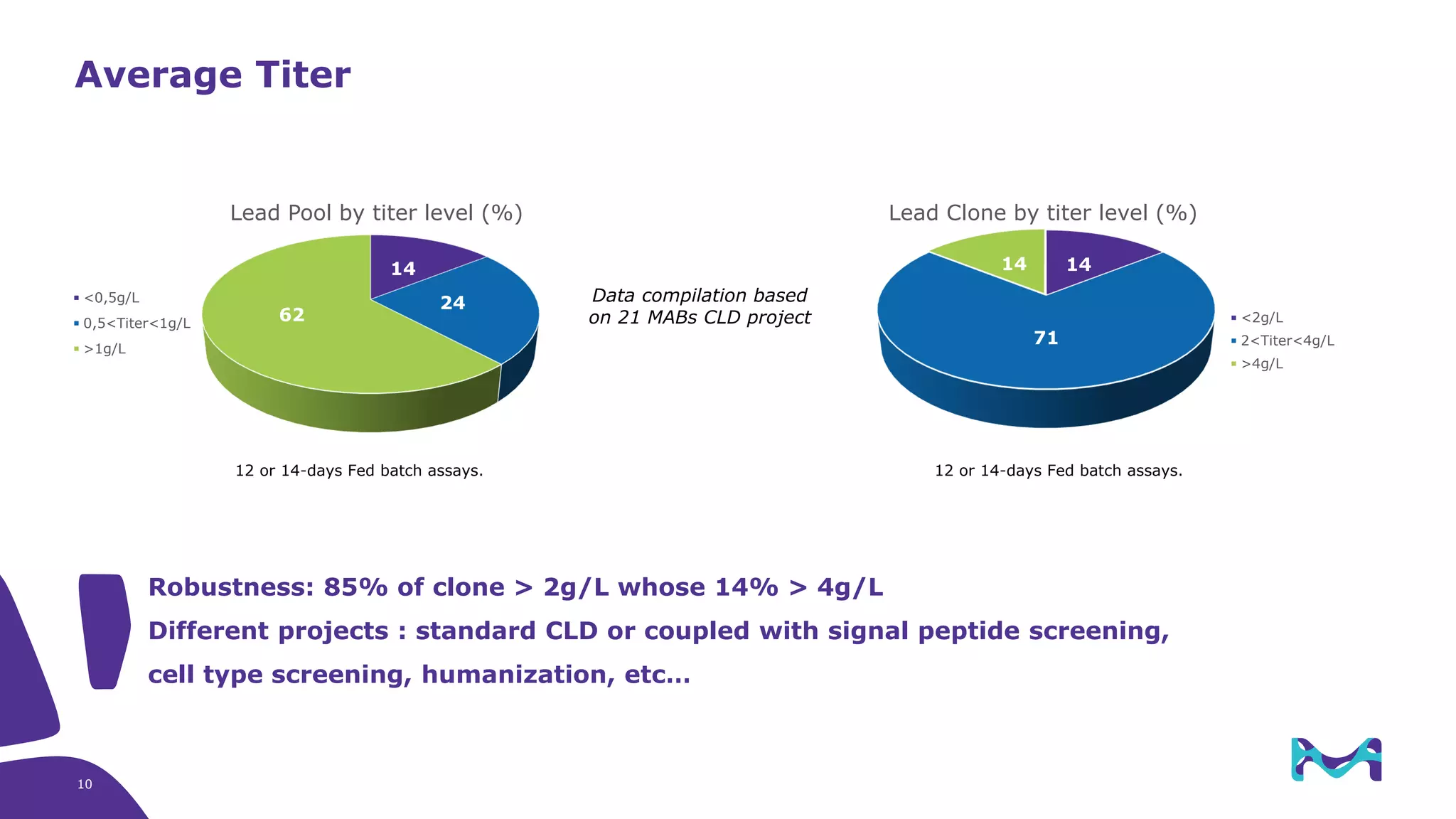
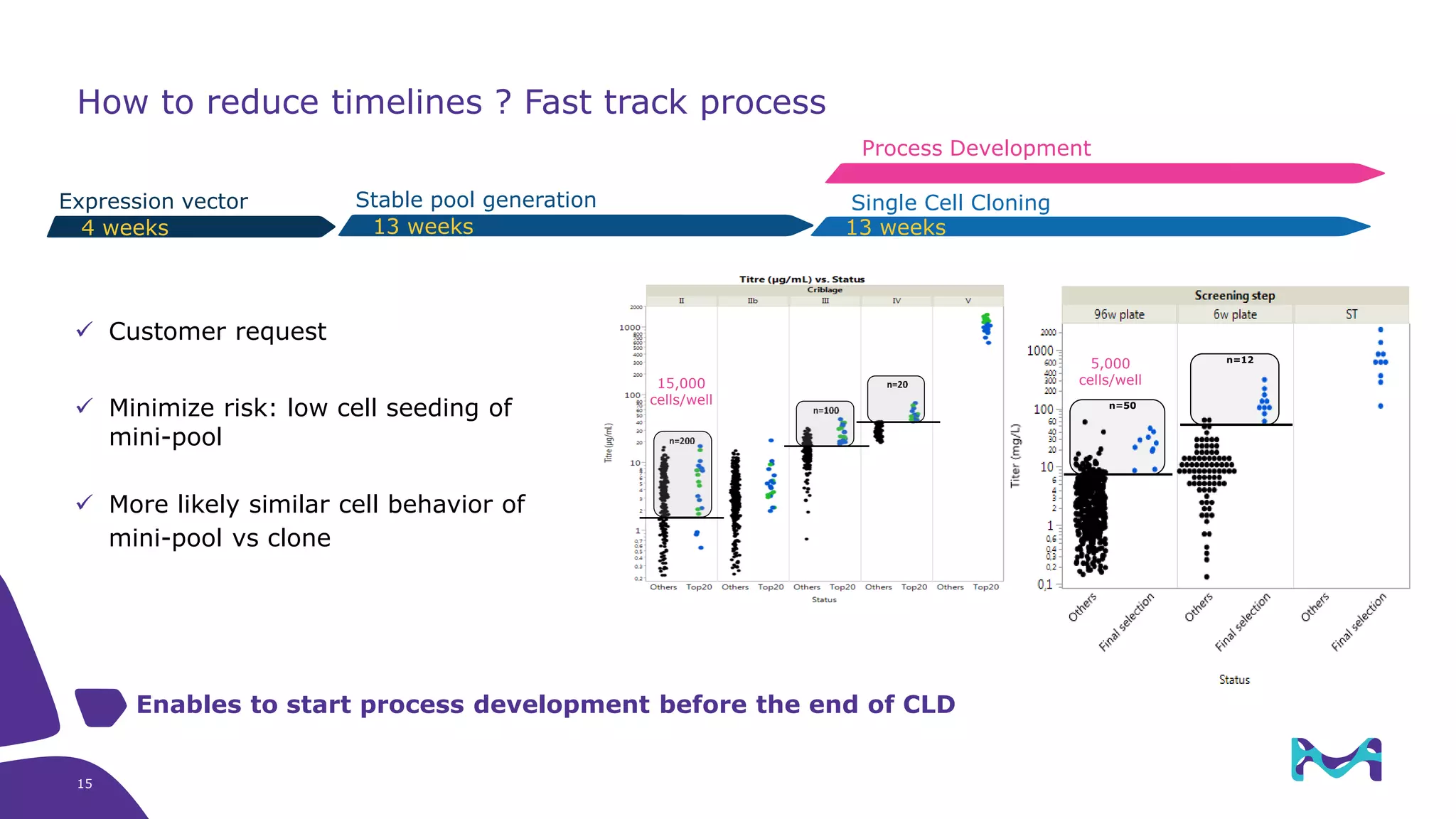
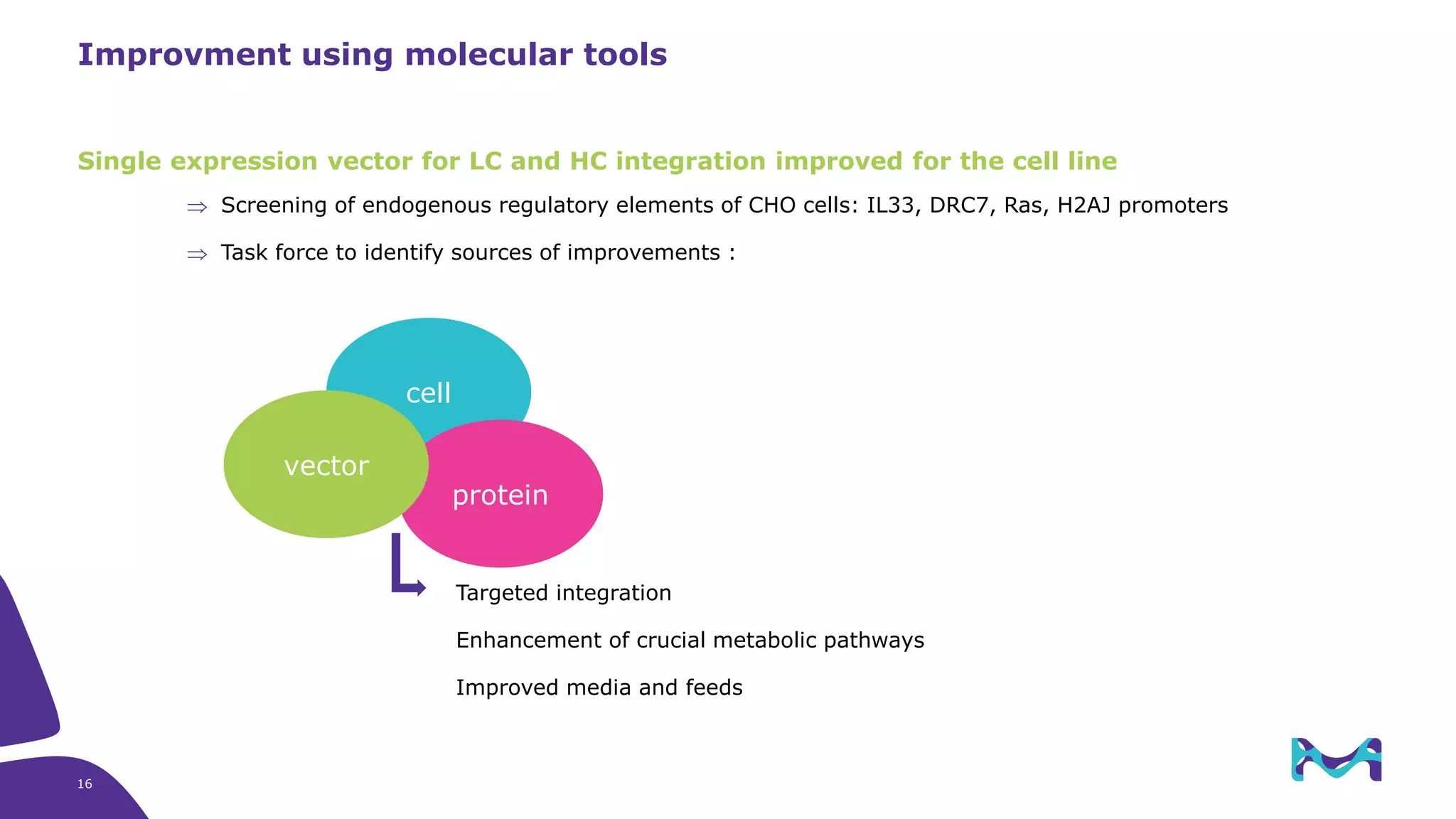
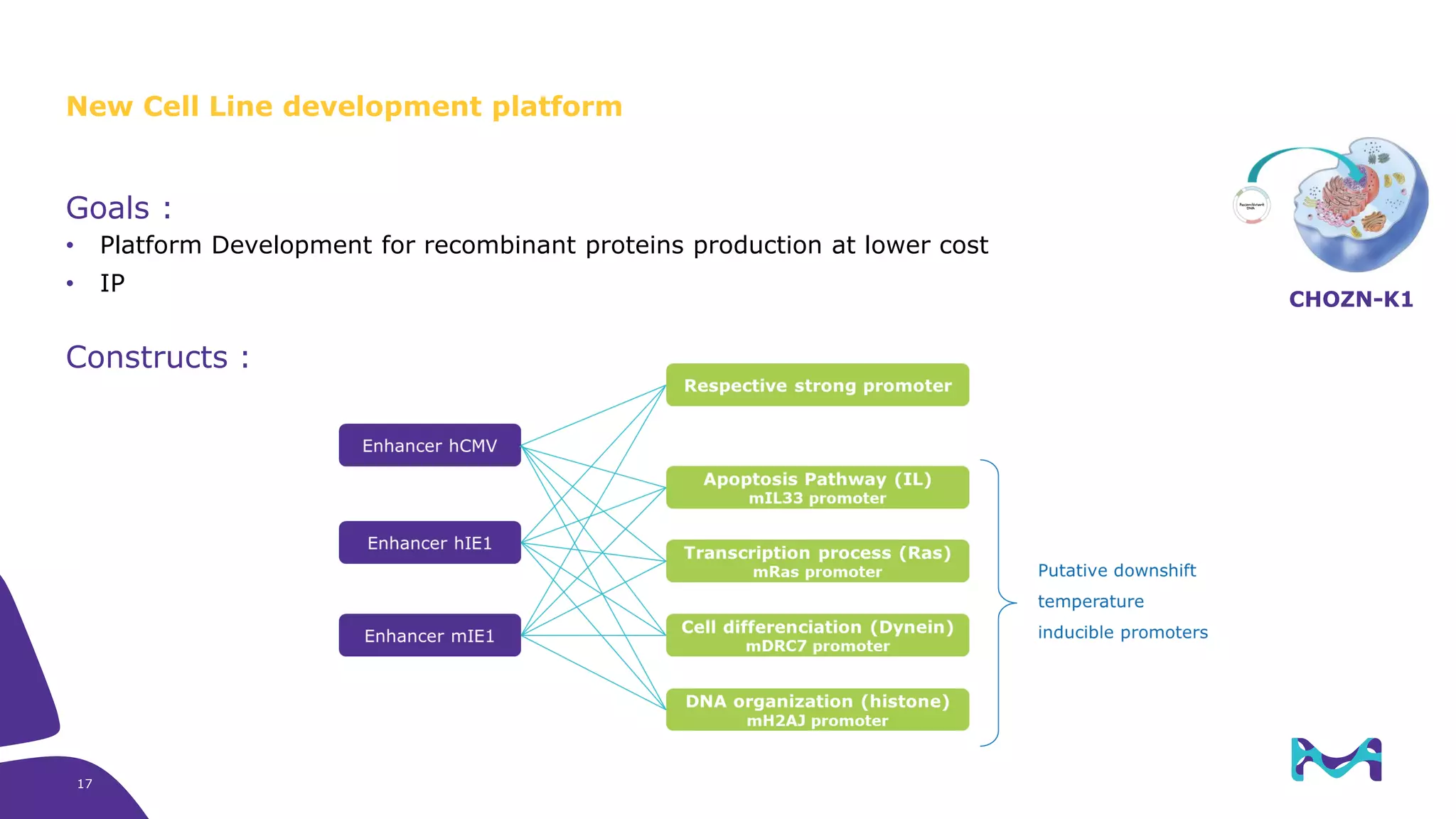
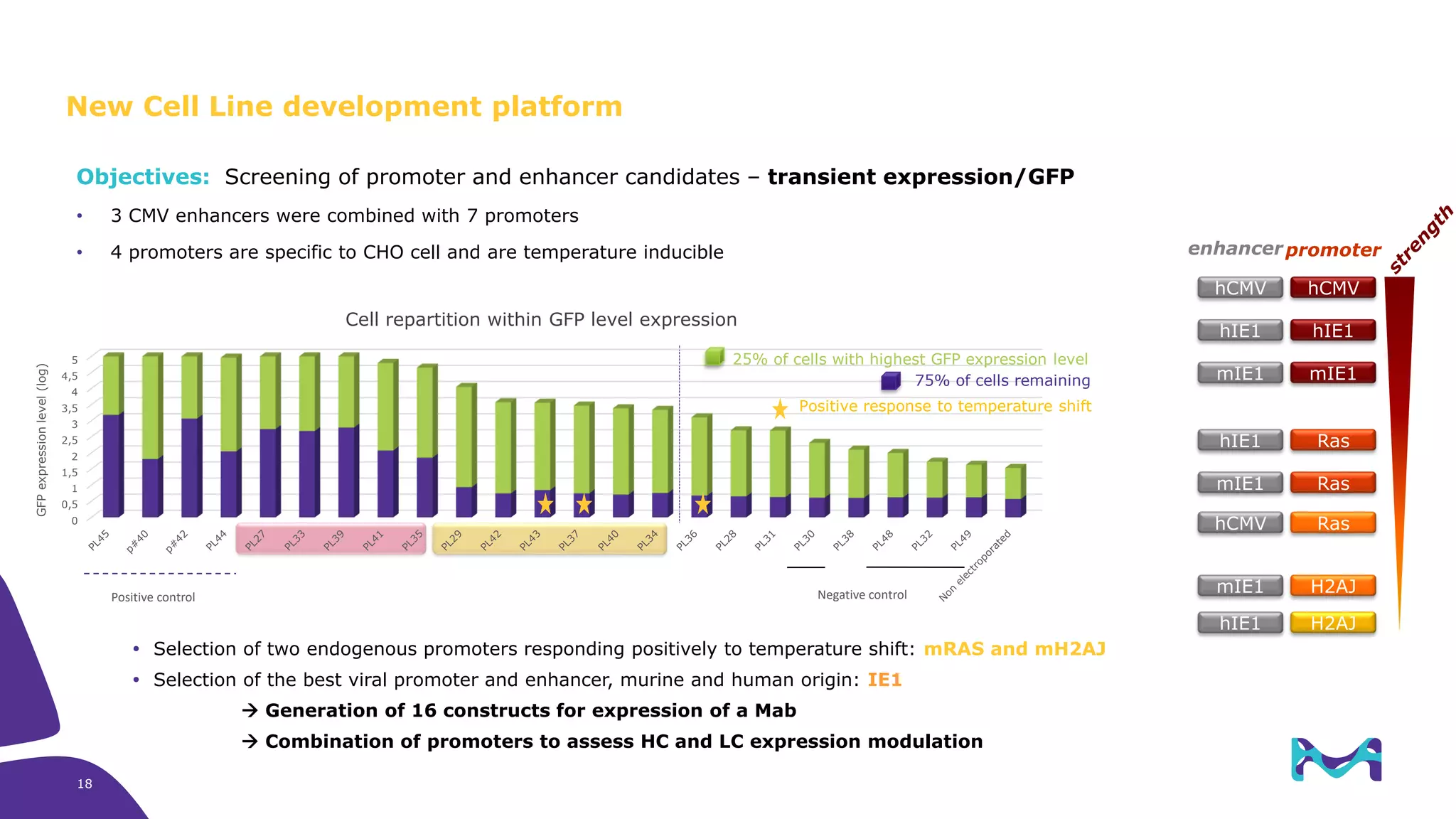
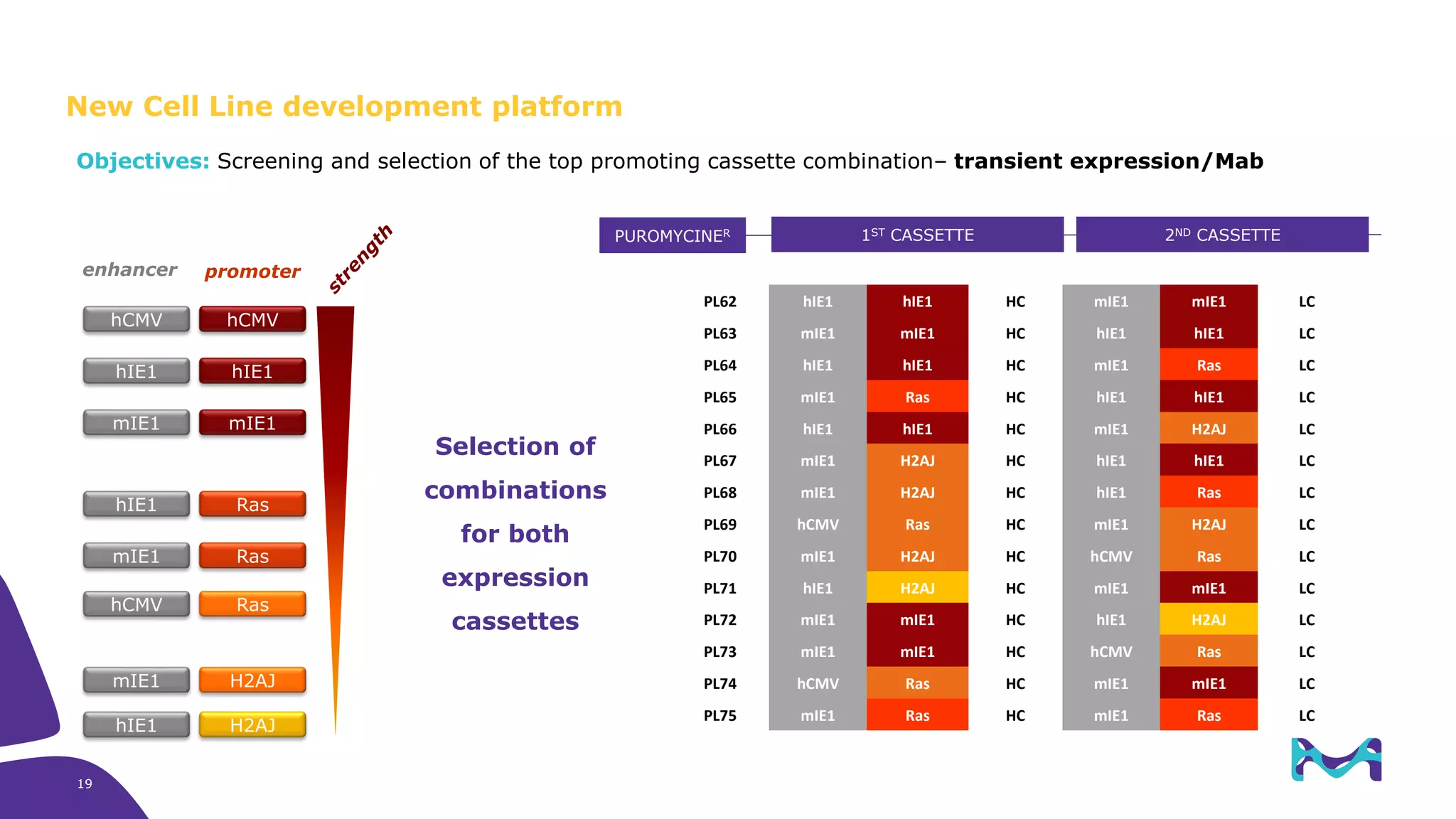
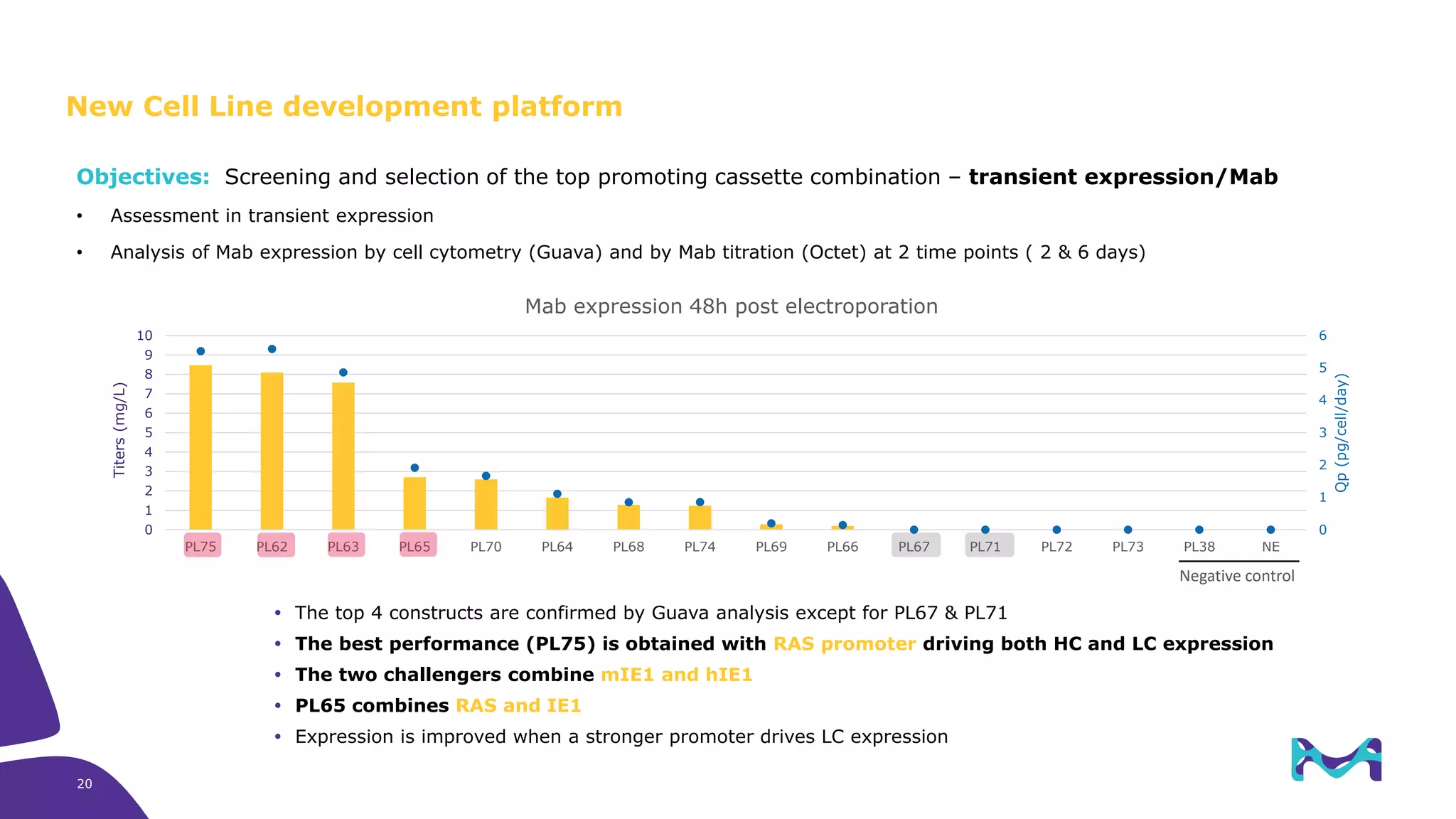
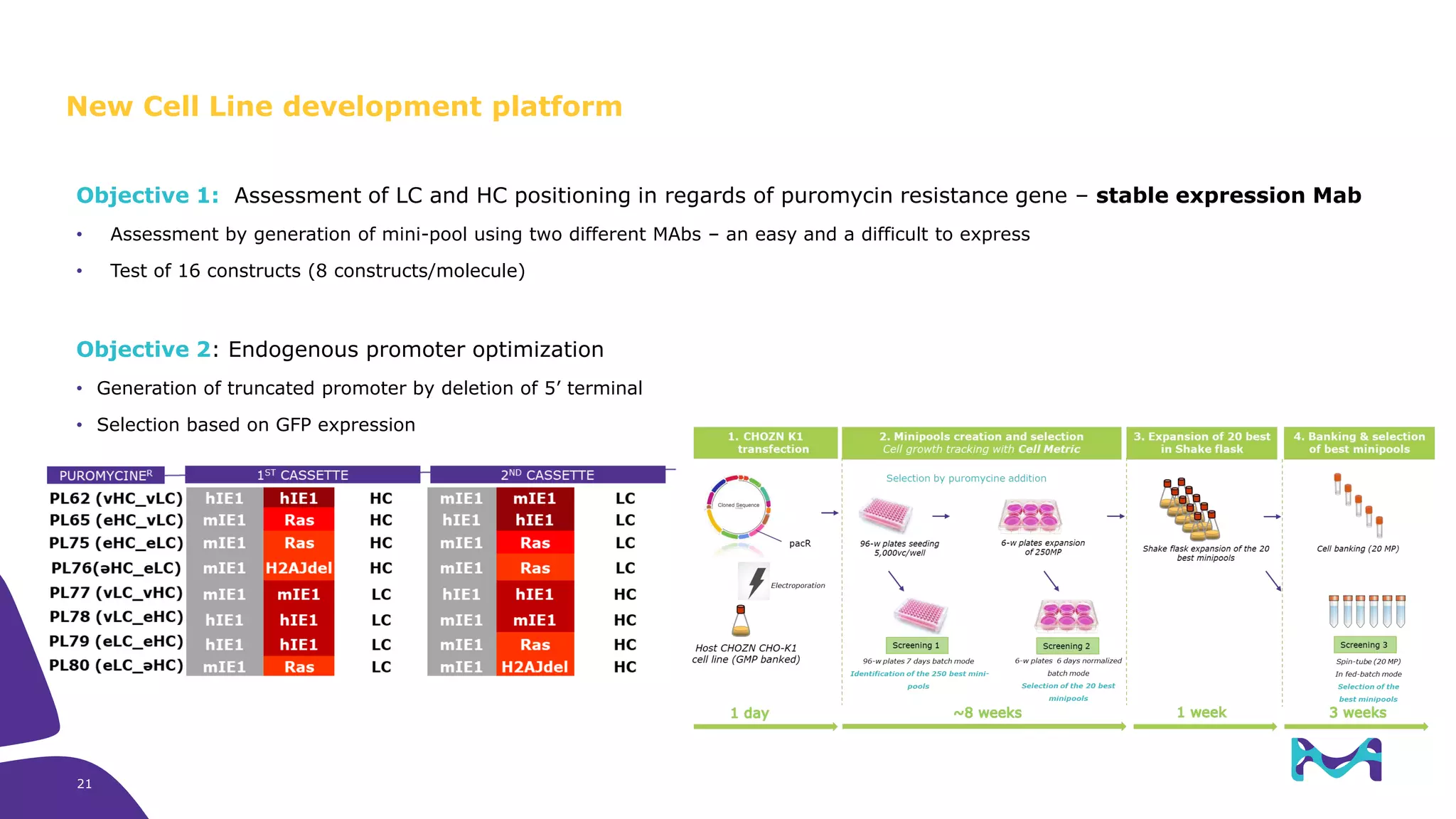
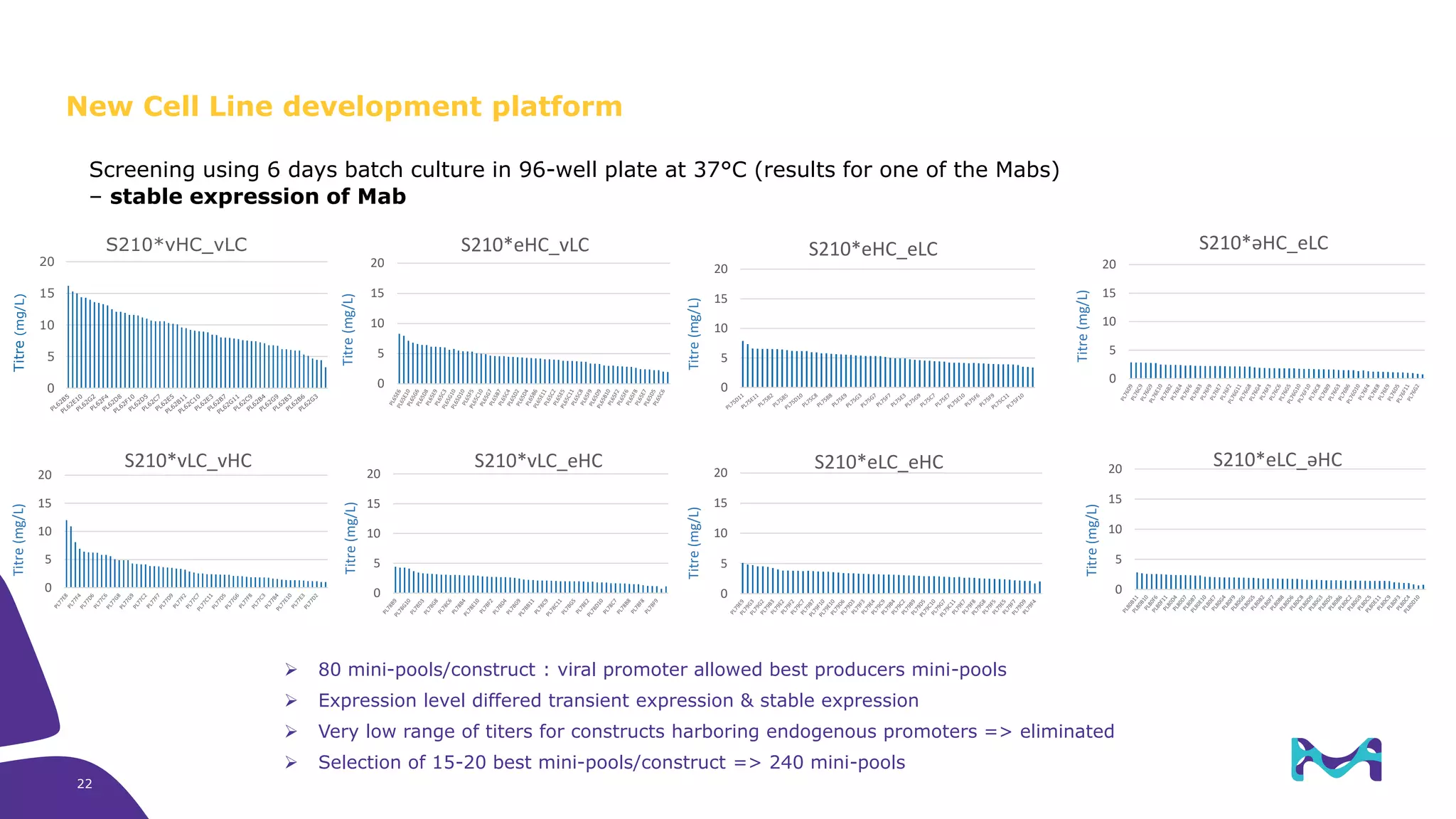
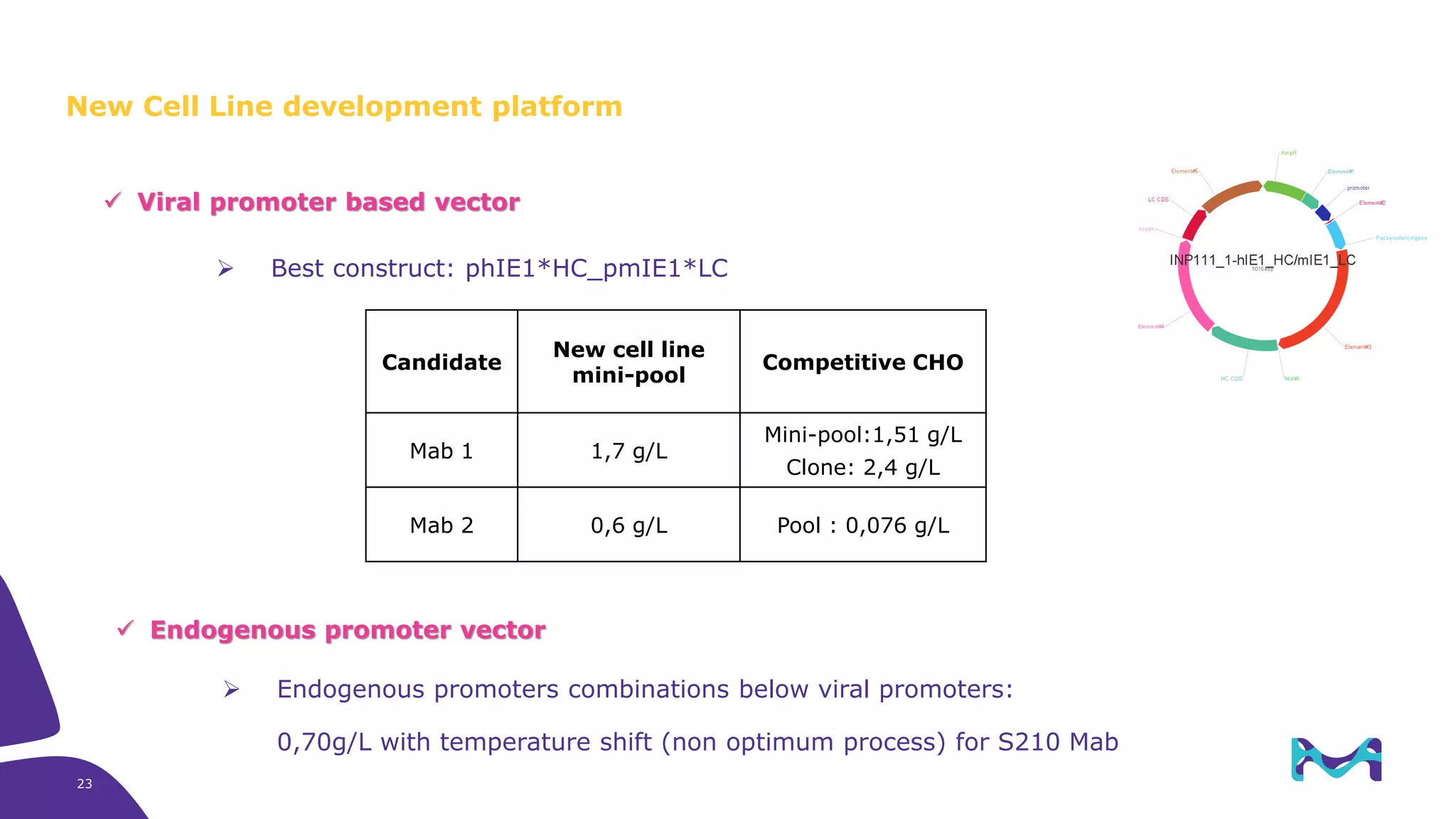
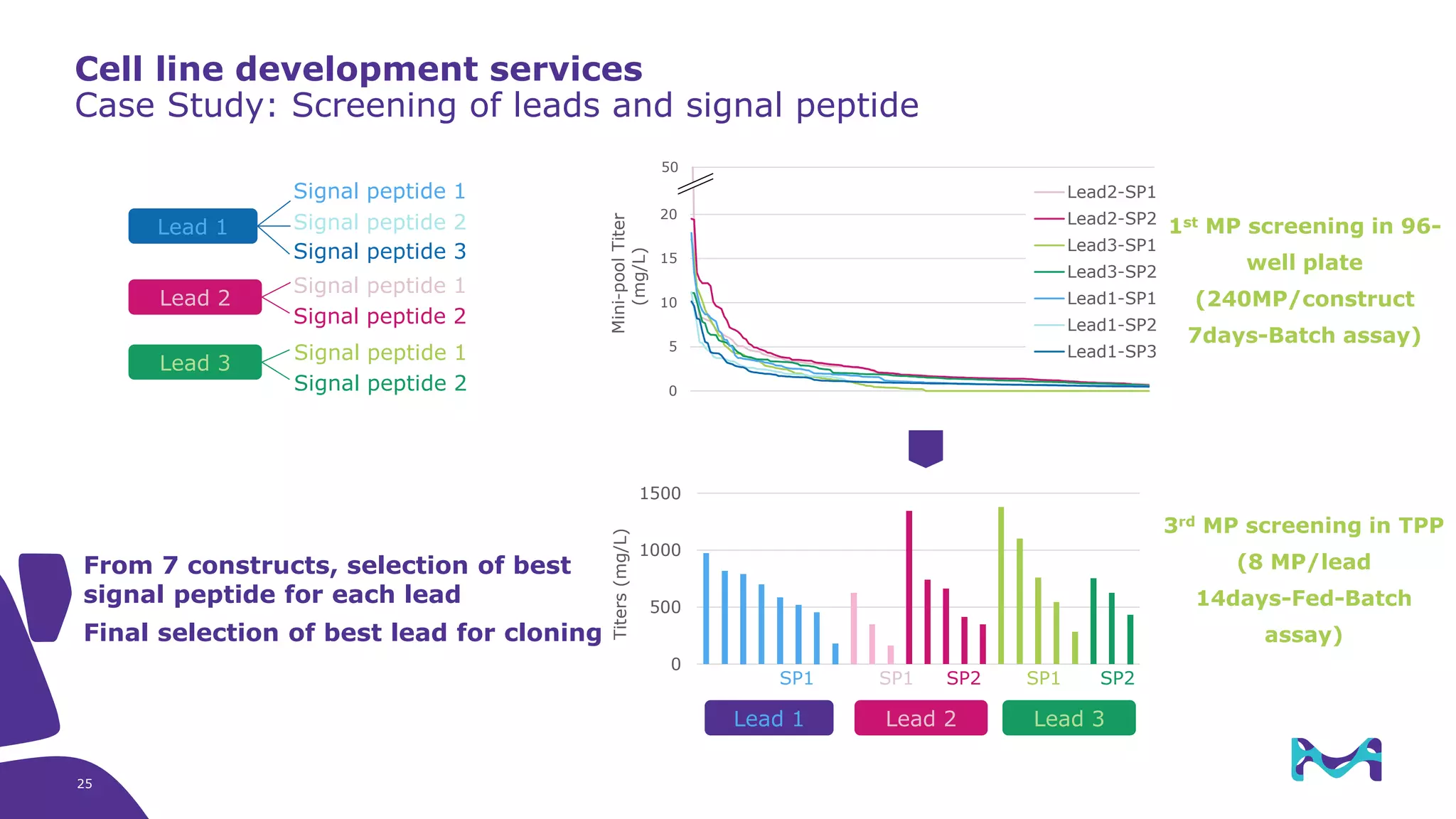
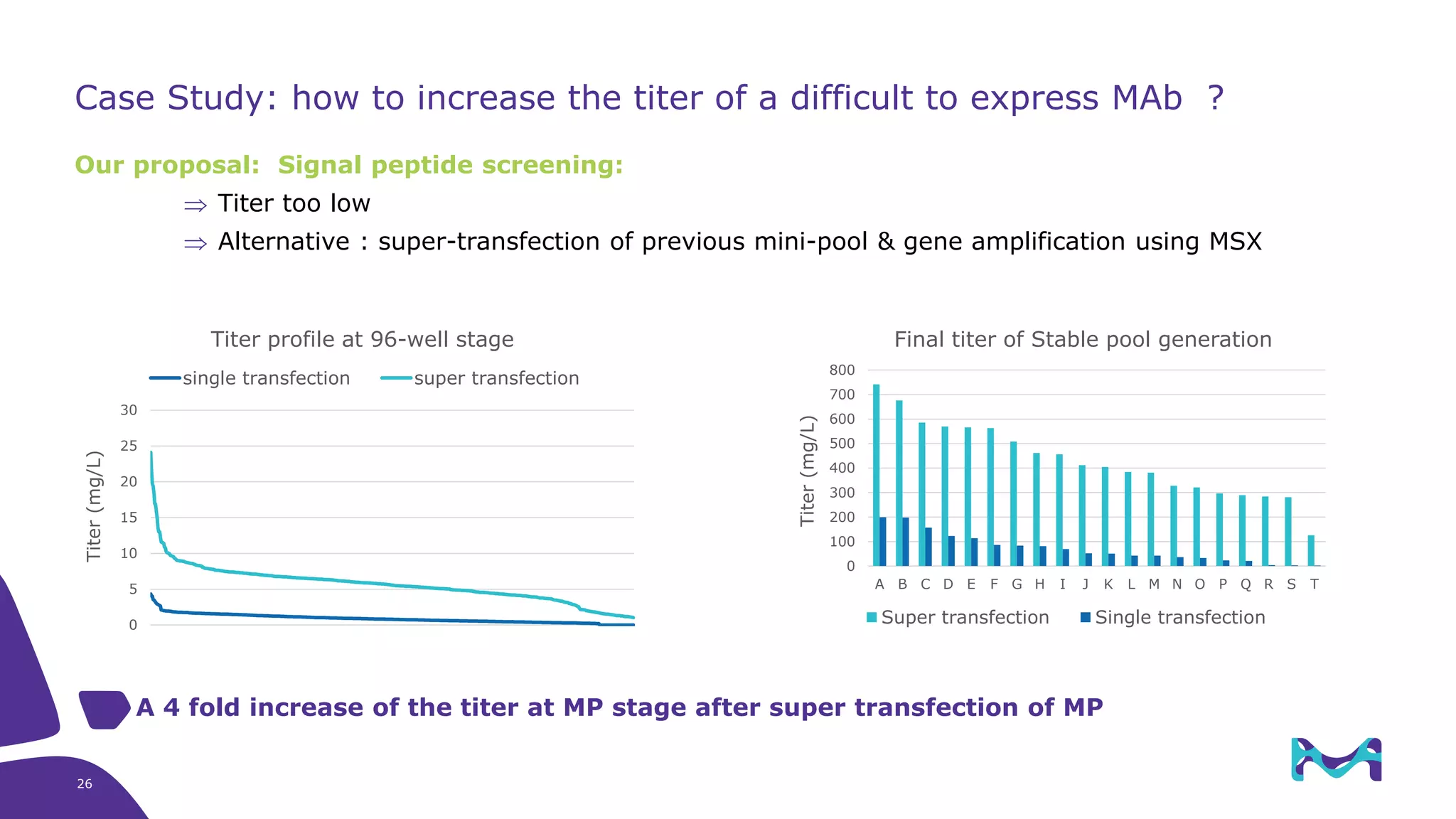
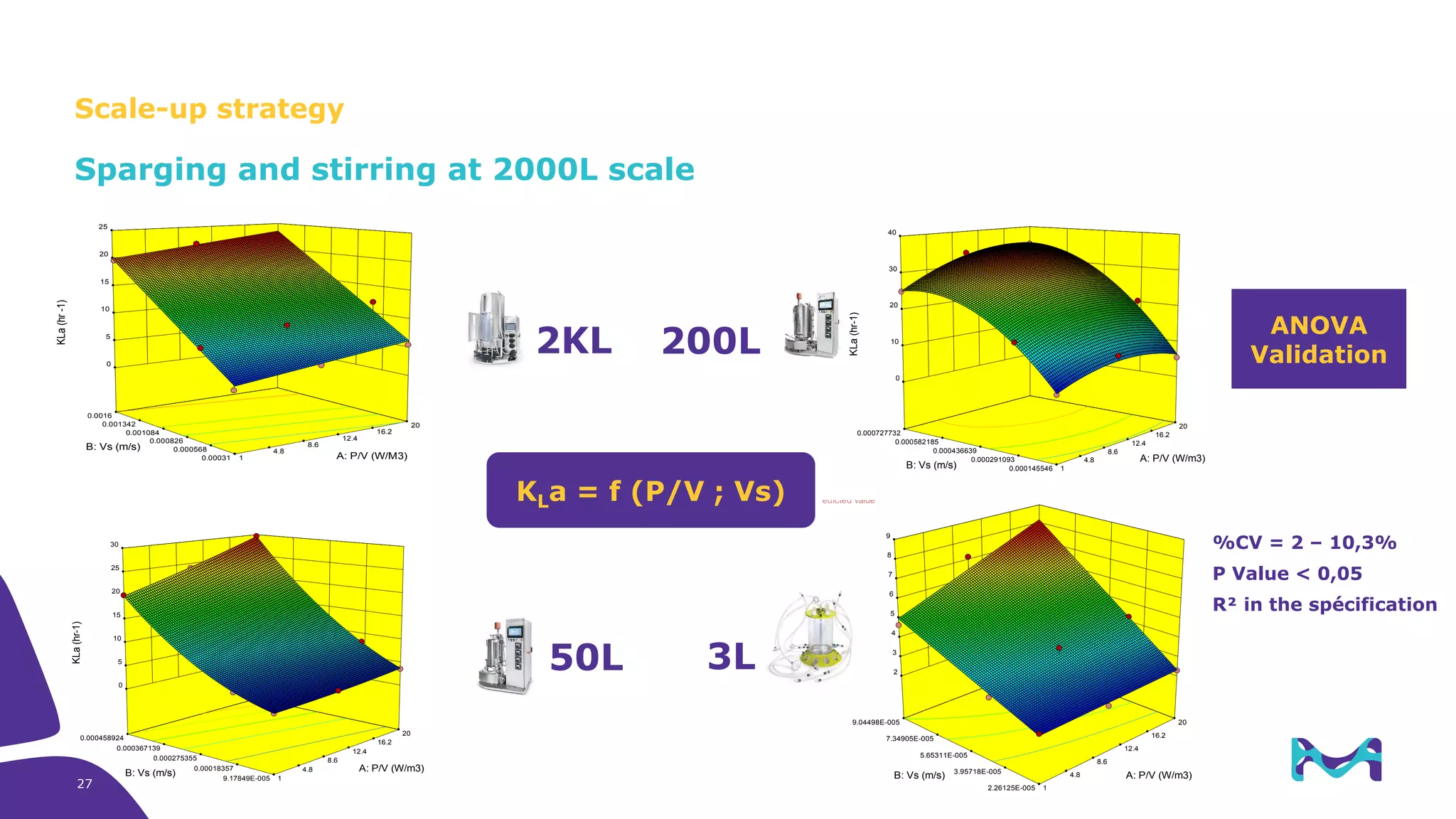
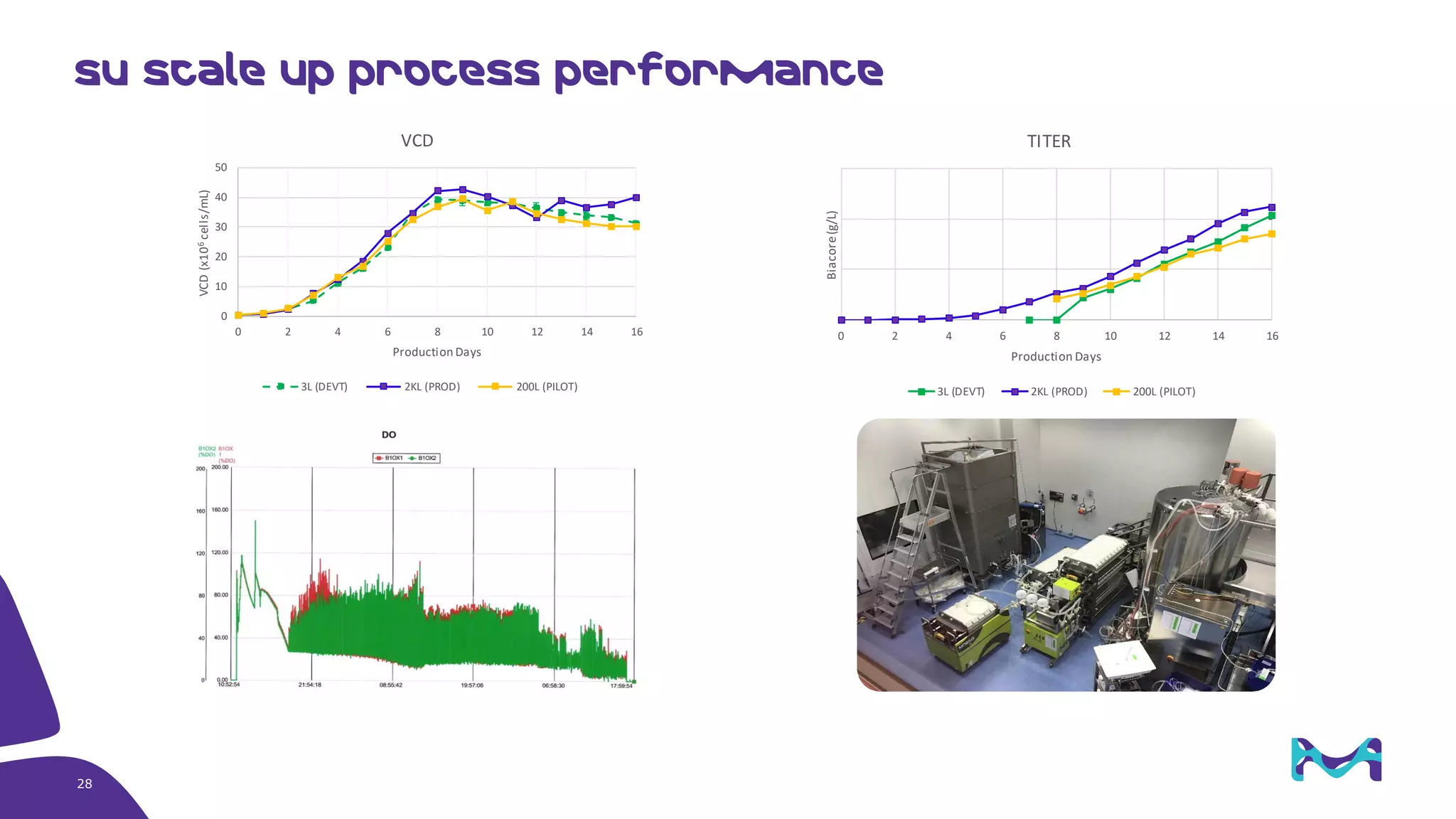
Merck KGaA provides cell line development and manufacturing services to support customers' biologics drug development programs, with over 30 years of experience developing over 250 cell lines. They have extensive expertise in cell line development, process development, analytical testing, and GMP manufacturing across multiple sites globally. The document highlights some of Merck KGaA's capabilities and case studies, including their approaches to optimizing cell line development techniques and processes like mini-pool screening and signal peptide selection to generate high producing cell lines.