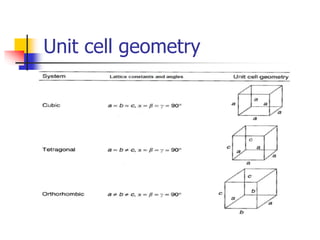
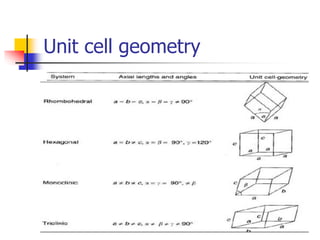
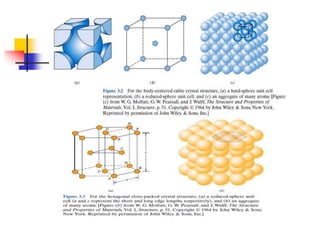
Crystals have orderly, repeating atomic patterns defined by a unit cell, whereas amorphous materials have disordered atomic distributions. Unit cells characterize crystal structures by their geometry and internal atom positions, and there are seven crystal systems classified by unit cell properties. Common metals form crystals with face-centered cubic, body-centered cubic, or hexagonal close-packed unit cell structures.