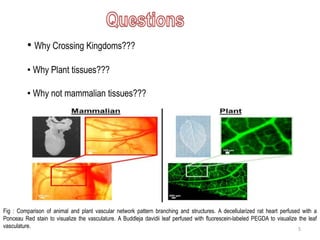
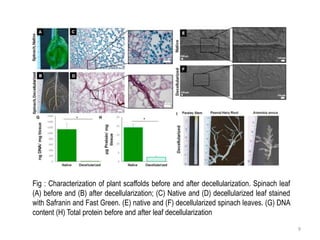
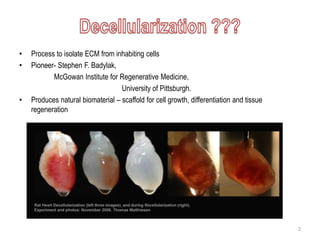
The document discusses methods for decellularizing and isolating extracellular matrices (ECM) from tissues, focusing on both mammalian organs and plant tissues, particularly spinach leaves. It highlights various physical, chemical, and enzymatic treatments for decellularization and demonstrates successful recellularization of human cells in decellularized plant structures, revealing similarities between plant and human vascular systems. The findings suggest potential applications in regenerative medicine using decellularized plant scaffolds.

![Figure : Combinations of agents used for the decellularization of (A) thin laminate tissues, (B) thicker
laminate tissues, (C) adipose tissues, (D) whole simple organs, (E) essential and complex organs .
3
• Physical Treatments : a. Freeze-thaw, b. Pressure, and c. Electric disruption [Non
thermal irreversible electroporation (NTIRE)].
•Chemical Treatment : a. Ionic detergents (SDS), b. Alkaline & acid treatments, and
c. Non-ionic detergents (Triton X-100, EDTA)
•Enzymatic Treatments : Lipase, Thermolysine, Galactosidase, Nuclease, Trypsin, Dipase,
and Collagenase
Perfusion and Immersion Decellularization Techniques](https://image.slidesharecdn.com/academicint-171120131747/85/Crossing-kingdoms-Using-decellularized-plants-as-perfusable-tissue-3-320.jpg)