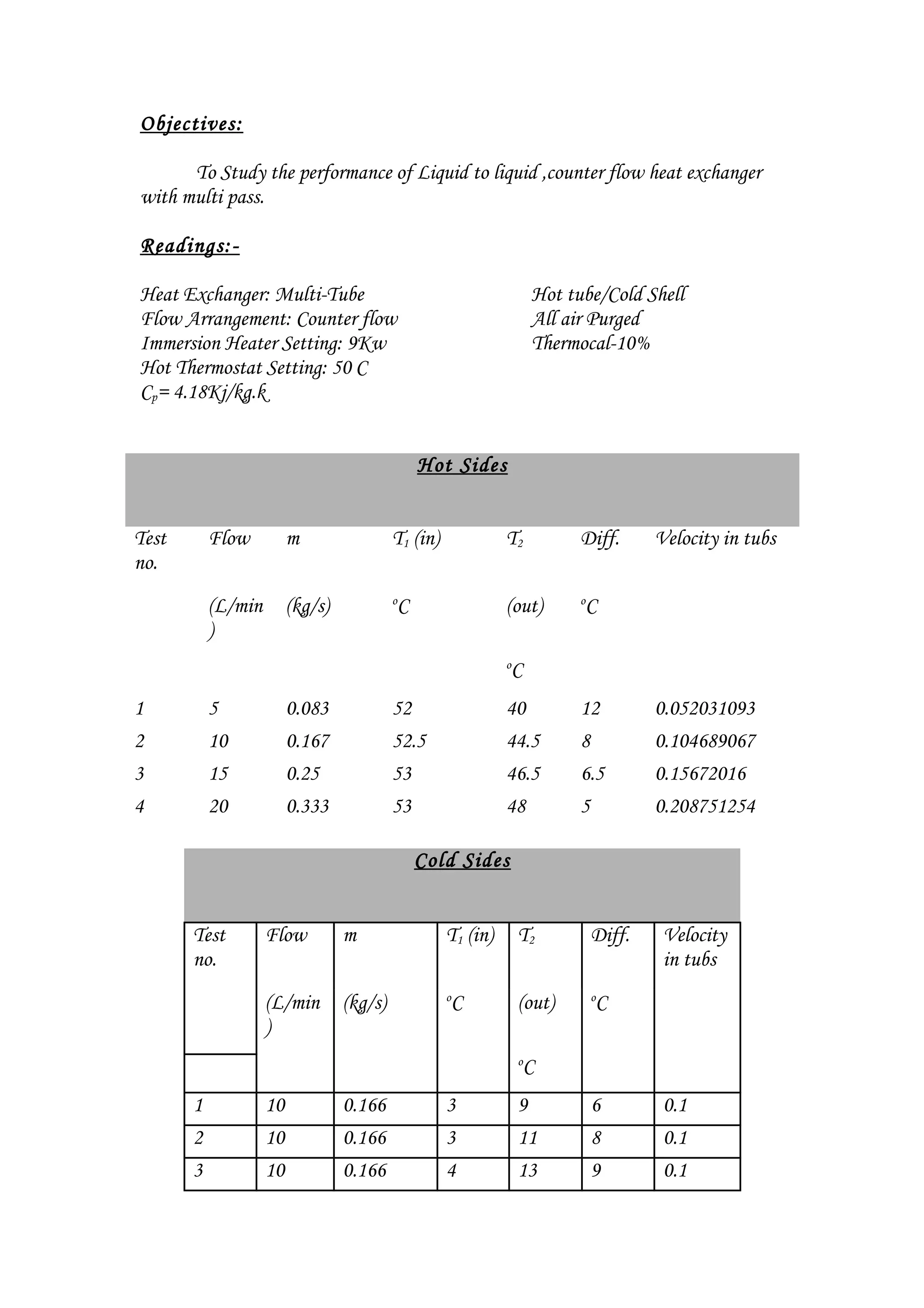
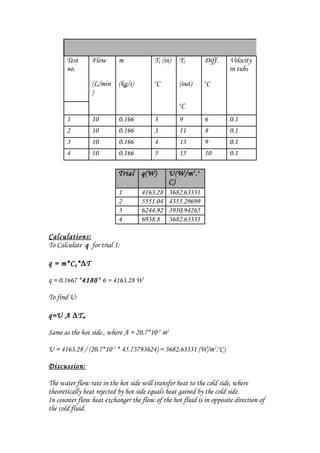
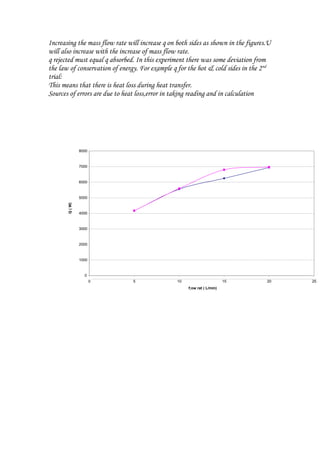
The document summarizes an experiment on a multi-pass counter-flow liquid-to-liquid heat exchanger. It includes readings from 4 trials measuring inlet and outlet temperatures on the hot and cold sides. Calculations were shown to determine the heat transfer (q) and overall heat transfer coefficient (U) for each trial. While q transferred was expected to be equal between the hot and cold sides, some trials showed a deviation, possibly due to heat loss. The heat exchanger performance improved with increasing flow rate as both q and U increased.