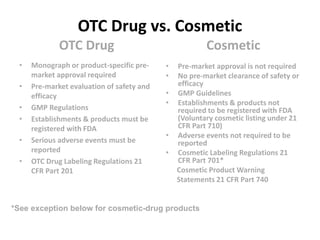
This document provides information on cosmetic labeling and claims requirements according to the FD&C Act. It defines cosmetics, lists examples of cosmetic product groupings, and provides examples of claims that could cause a cosmetic product to be considered misbranded or regulated as a drug or medical device. It also discusses differences between OTC drugs, cosmetics, and medical devices and issues related to products that are both cosmetics and drugs.