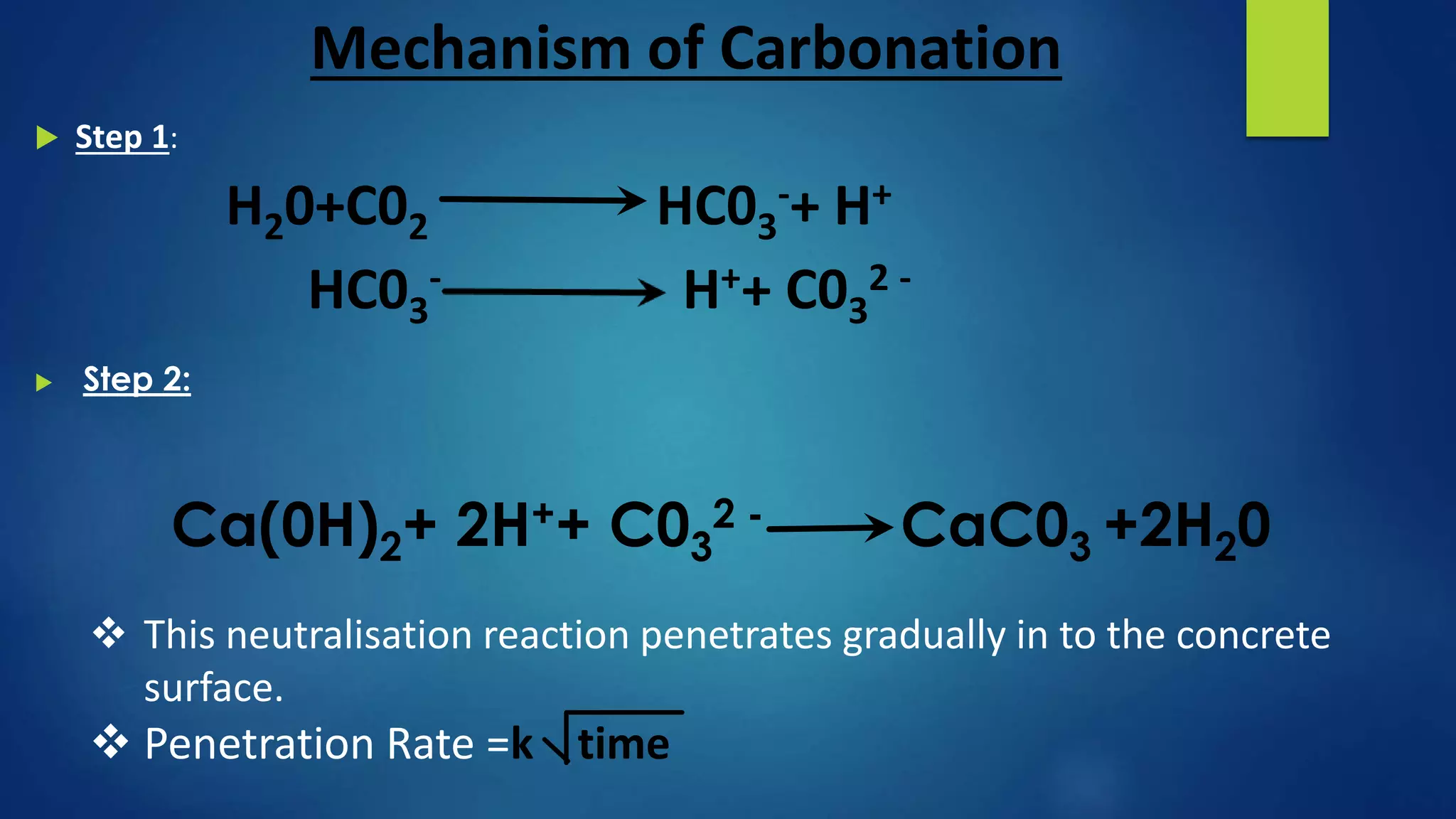
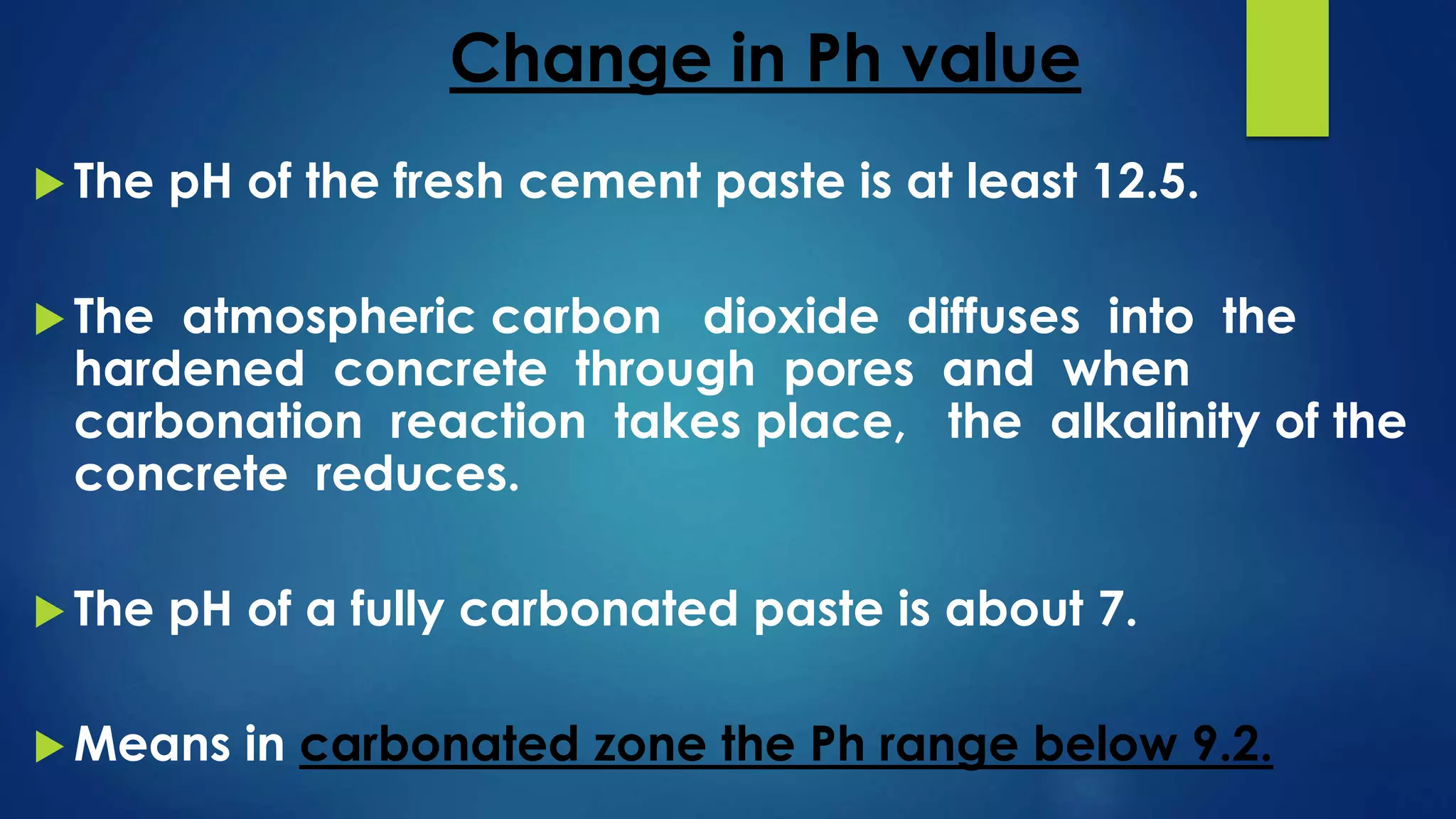
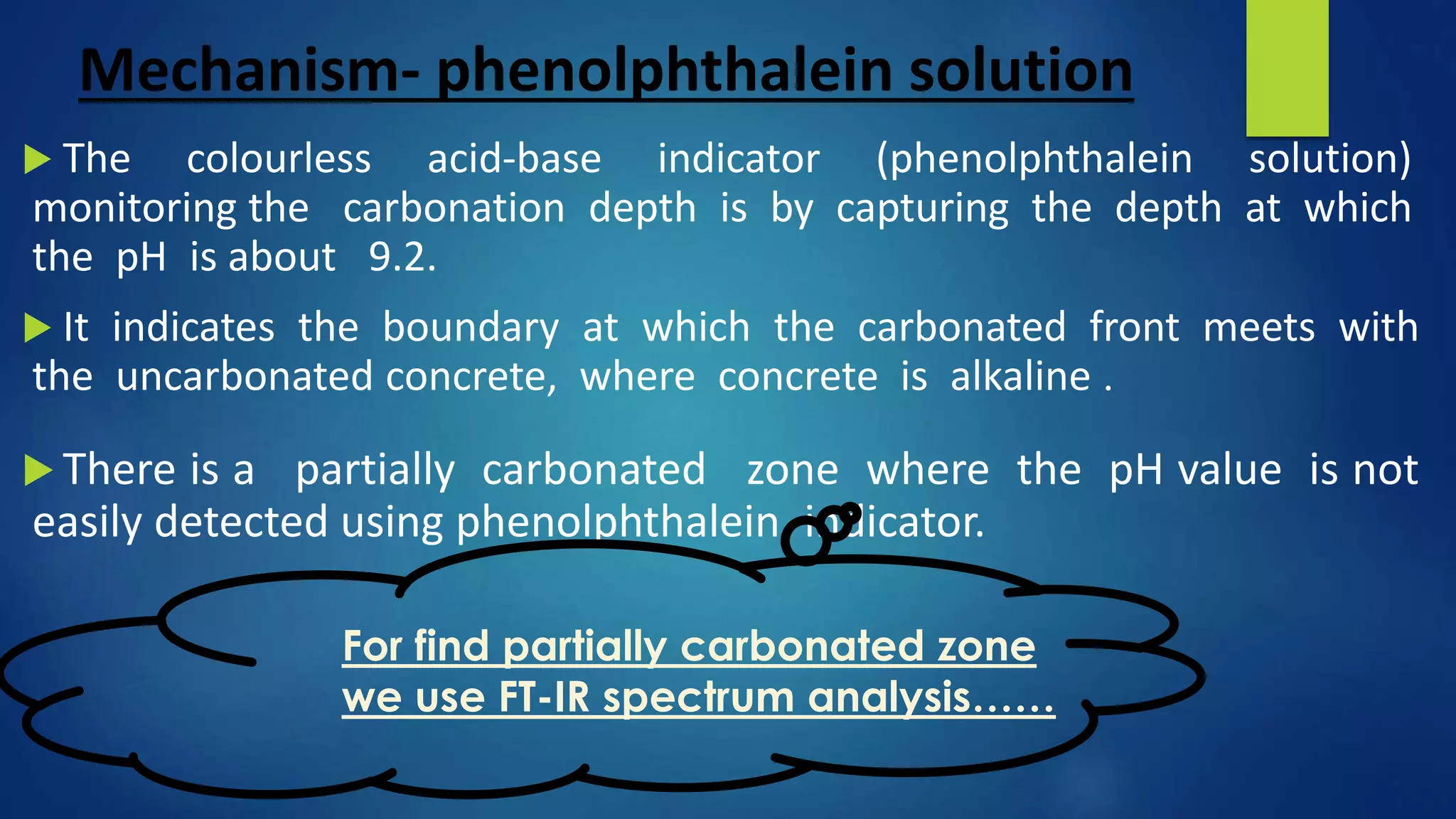
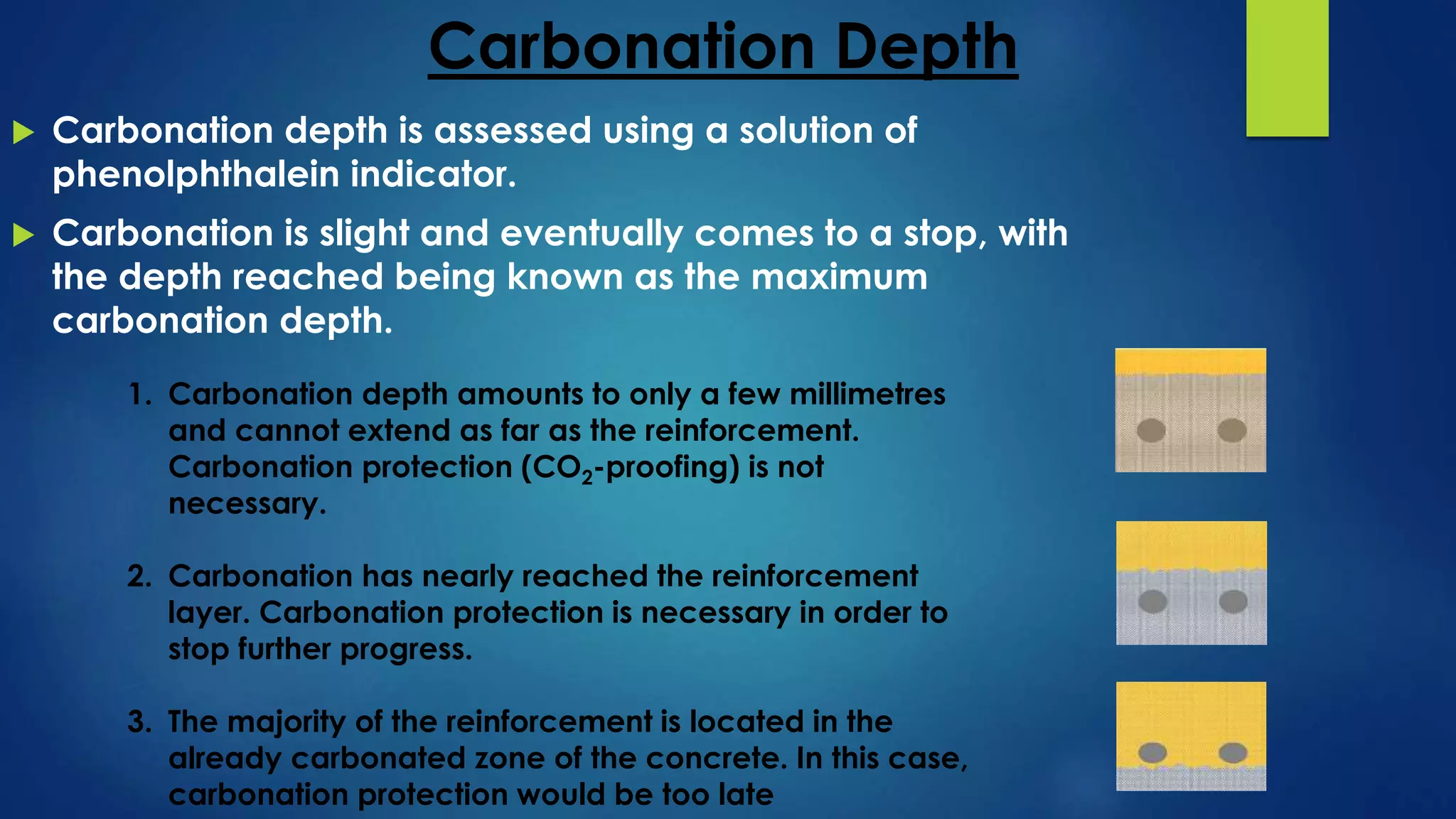
Carbonation is a chemical process where atmospheric carbon dioxide reacts with compounds in concrete, like calcium hydroxide, to form calcium carbonate, reducing the pH of concrete. This reaction exposes the reinforcing steel to corrosion. The depth of carbonation depends on factors like CO2 concentration, concrete pore structure, and relative humidity. Carbonation can be tested by using phenolphthalein solution, which changes color at pH 9.2, indicating the boundary between carbonated and uncarbonated concrete. Protective coatings can prevent further carbonation by providing a barrier to water and CO2 ingress.