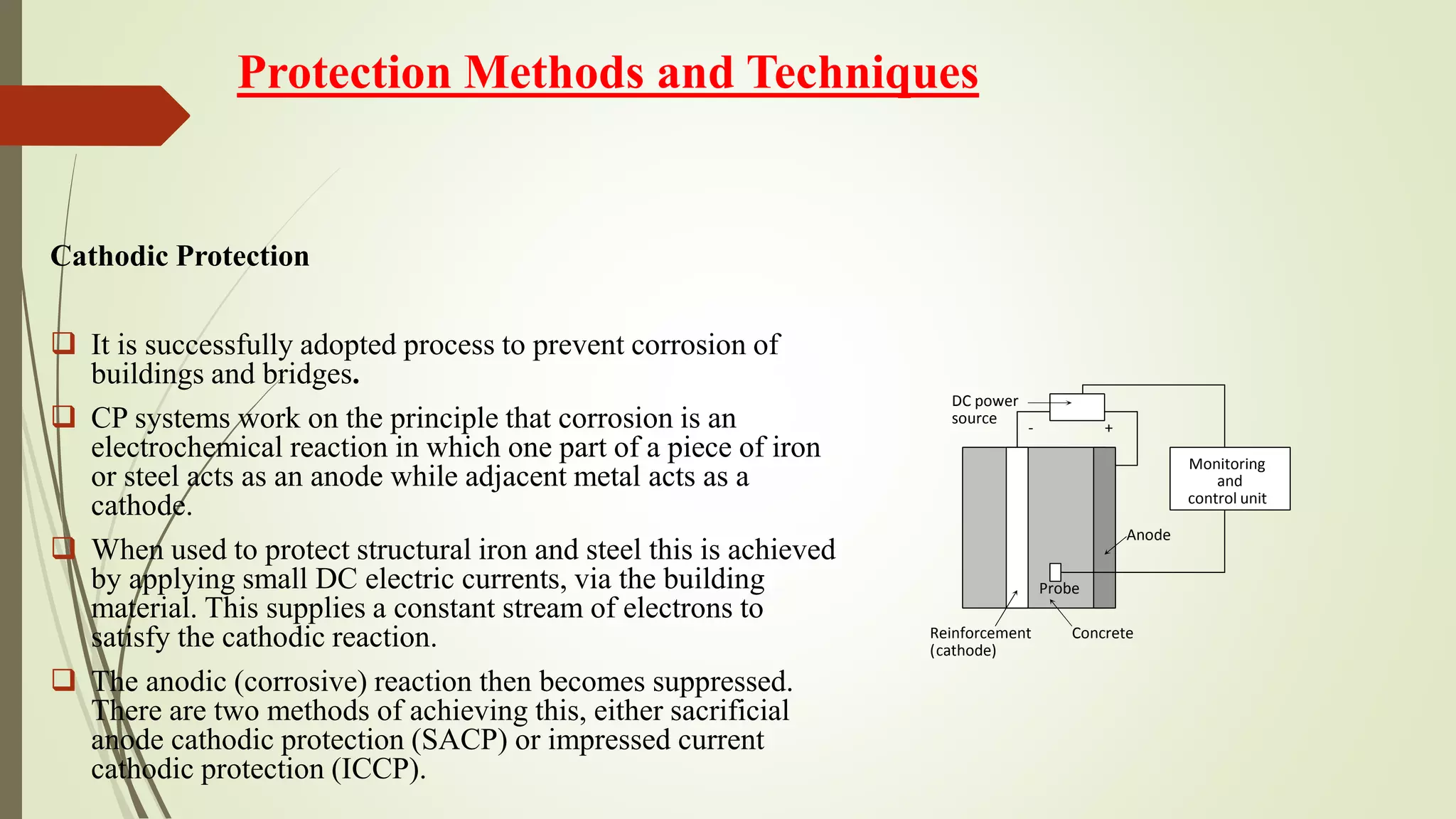
This document discusses corrosion in reinforced concrete structures, including its history and causes. It provides details on several bridge failures due to corrosion, including the collapse of the Mianus River bridge in 1983 and the Silver Bridge in 1967. The document also discusses experimental work on corrosion, including the effects of strain level, corrosion duration, and stress. It notes that carbonation and chloride ions can lead to corrosion by lowering the pH and attacking the steel. Permeability of concrete and thermal movement are identified as key causes of cracks in structures. Protection techniques like coatings and cathodic protection are also mentioned.