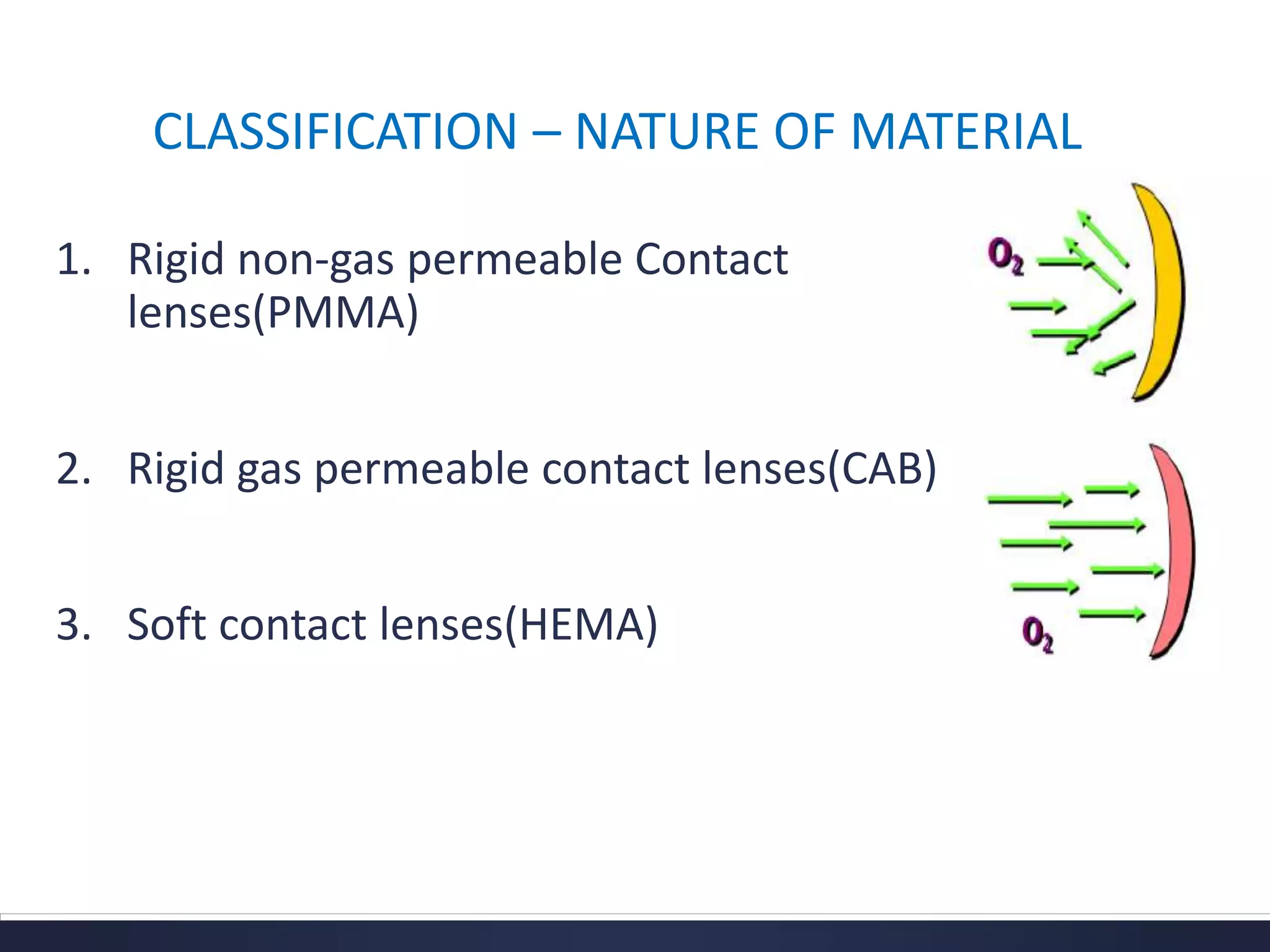
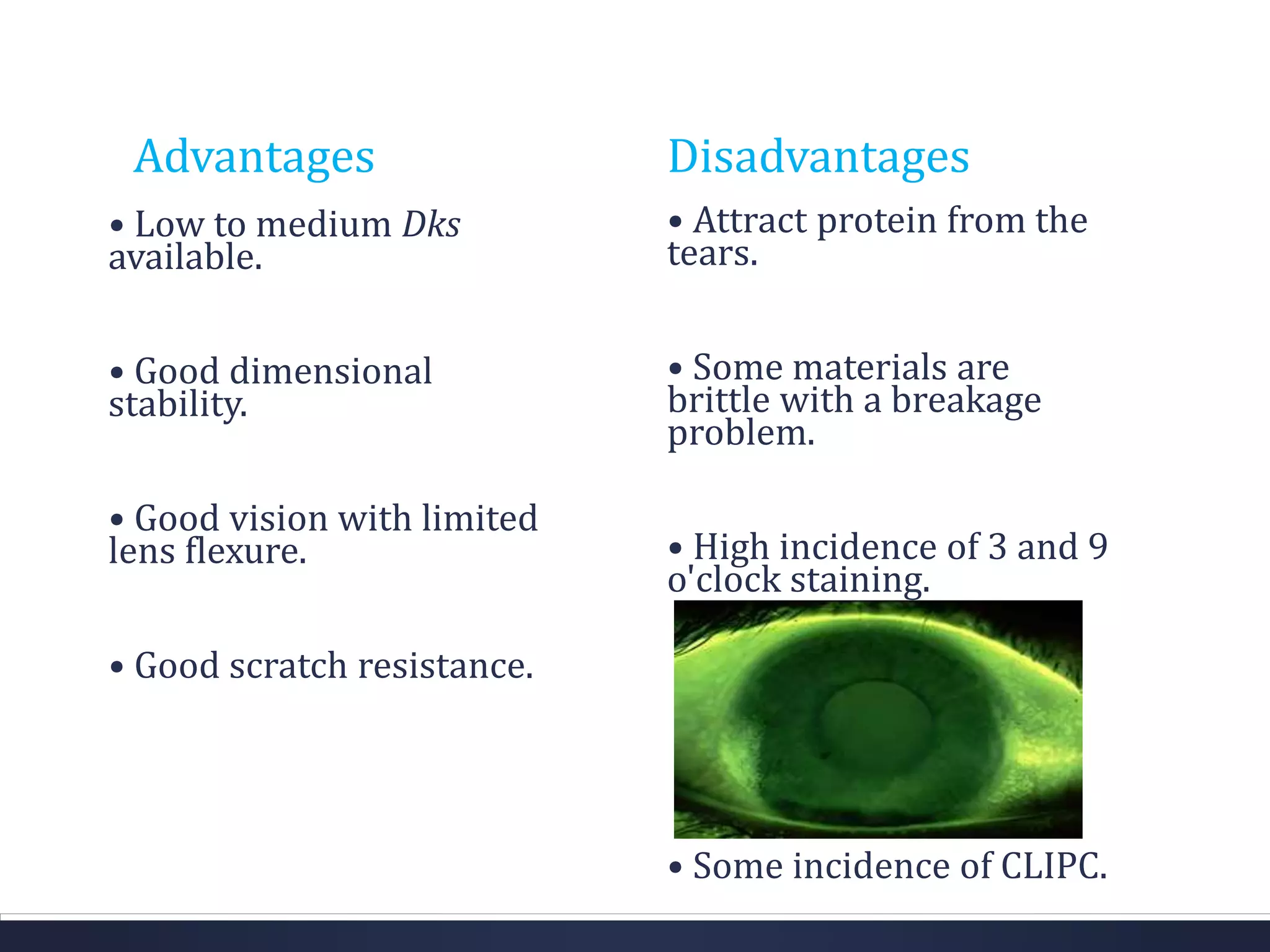
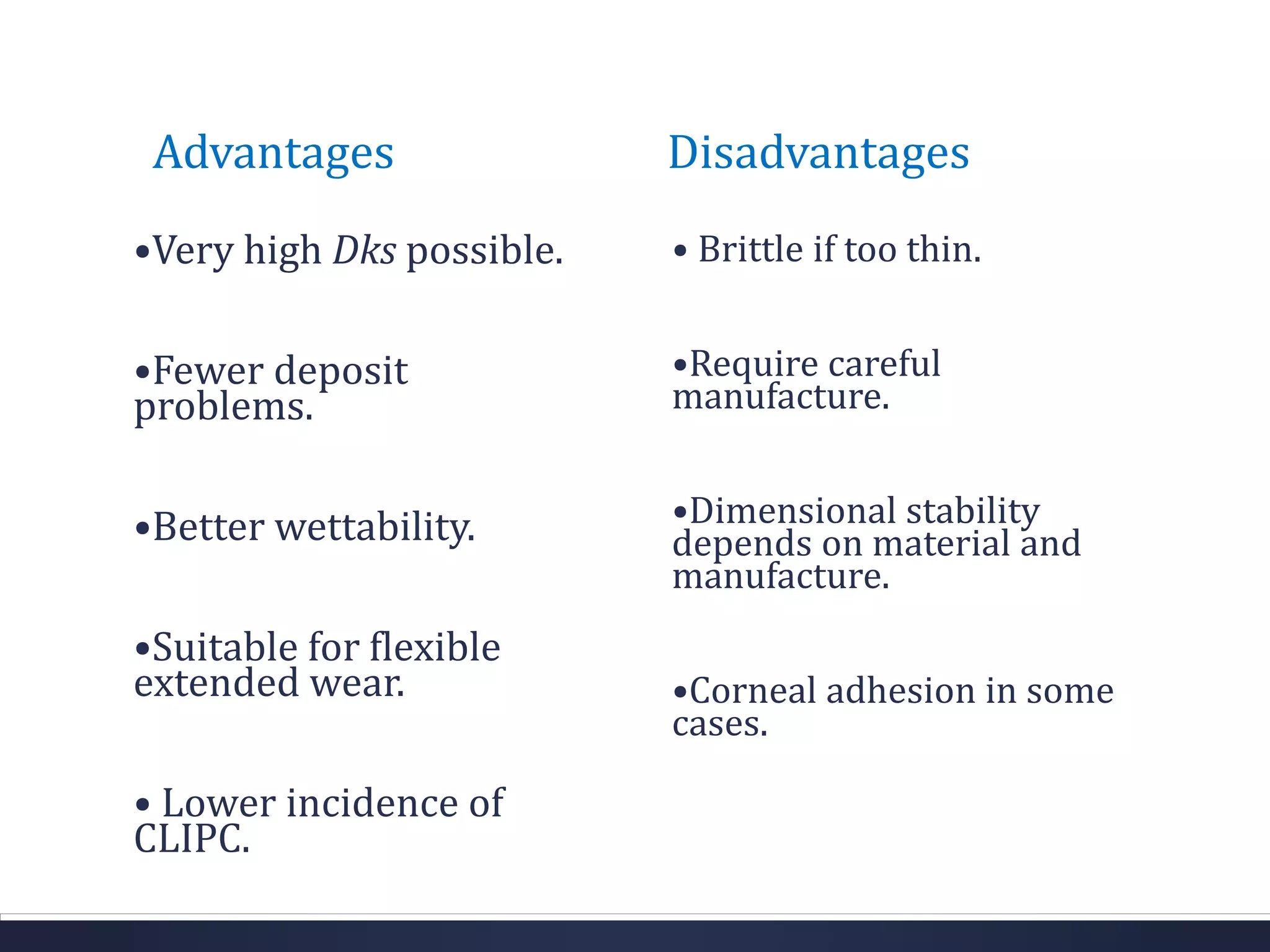
This document discusses the history and materials used for contact lenses. It begins with a brief history of contact lens development starting in the 1500s. It then covers the classification and properties of various contact lens materials, including rigid non-gas permeable, rigid gas permeable, and soft contact lenses. The ideal properties for lens materials and examples like PMMA, silicone, and HEMA are summarized. Finally, it discusses contact lens solutions and their functions in wetting, cleaning, storage and rewetting lenses.