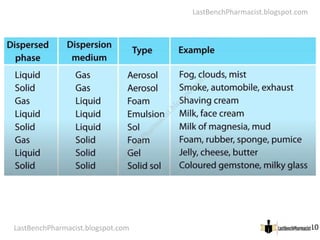
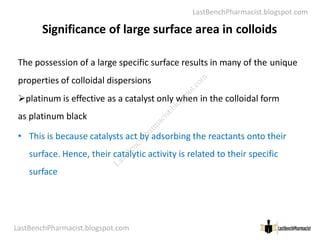
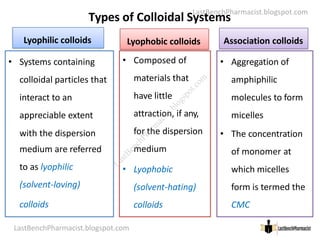
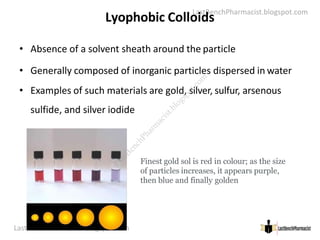
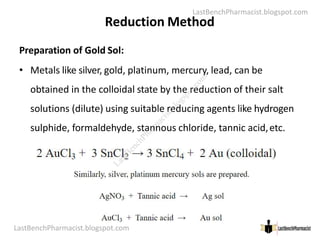
The document serves as a lecture outline on colloids, detailing learning objectives and components of dispersed systems, while classifying colloids into types based on particle size: molecular, colloidal, and coarse dispersions. It also explains the significance of surface area in colloidal systems, types of colloid interactions (lyophilic and lyophobic), and methods for preparing both types of colloids. Various preparation techniques such as dispersion and condensation methods are discussed, along with examples and applications of colloids in different media.